Abstract
Purpose
Acinetobacter baumannii (A. baumannii) has emerged as a major cause of nosocomial pneumonia and sepsis in seriously ill patients. Multidrug-resistant A. baumannii (MDRAB) is increasing in frequency, and the management of it's infections is consequently difficult. Therefore, tigecycline is considered to be the drug of choice for MDRAB treatment. The aim of our study was to evaluate the microbiological eradication and clinical effectiveness of tigecycline against MDRAB in seriously ill patients, including patients with ventilator-associated pneumonia (VAP).
Materials and Methods
We conducted a retrospective study including patients with A. baumannii infections who were treated with tigecycline between April 1, 2009 and March 31, 2010. We treated 27 patients with tigecycline for MDRAB infections.
Results
The mean age of patients was 66.2 years, and 20 (74.1%) patients were male. The median length of stay at hospital was 74.6 days. MDRAB was eradicated from the site of infection in 23 cases (85.2%), however, only 17 cases (63.0%) showed positive clinical responses. Overall, an in-hospital mortality rate of 51.9% was observed, and 4 cases of death were attributable to sepsis. The combination therapy showed better clinical and microbial success rates than the monotherapy without significant difference.
Conclusion
We observed the relatively low clinical success rate although the microbial eradication rate was high, probably due to superinfections in VAP and bacteremia. We suggest that clinicians should limit tigecycline monotherapy for MDRAB infection in critically ill patients, until large controlled clinical trials should be conducted.
Keywords: Acinetobacter baumannii, bacteremia, multidrug resistance, tigecycline, ventilator-associated pneumonia
INTRODUCTION
Acinetobacter baumannii (A. baumannii) has emerged as a major cause of nosocomial pneumonia [particularly ventilator-associated pneumonia (VAP)], bloodstream infection and sepsis in immunocompromised and seriously ill patients.1,2 Mortality of critically ill patients with A. baumannii infections is high.3-7 Furthermore, multidrug-resistant A. baumannii (MDRAB) is increasing in frequency and it's management can consequently be difficult.4,8-10 Carbapenems have become the therapy of choice for serious A. baumannii infections, however, carbapenem-resistant organisms are also becoming more common.11-14
Many MDRAB strains remain susceptible to tigecycline in vitro.15 However, reports of MDRAB are increasing, as confirmed by a European study group16 and an American study group17 that showed 51-67% resistance to quinolones and 40-63% resistance to third generation cephalosporins. Moreover, carbapenem resistance has increased to 12-47%. Resistance to colistin is also reported to be up to 22%, and the nephrotoxicity of colistin limits its usage in many seriously ill patients with renal failure.16,17 Therefore, when these drugs are not effective in MDRAB infections, tigecycline is considered as the drug of choice.
Even though some observational studies have shown that VAP caused by MDRAB was an important indication for the use of tigecycline,18-21 the role of tigecycline in VAP control is still uncertain. Extended clinical experience in critically ill patients with MDRAB infections is poor, and most of the available publications are retrospective with small numbers of patients or with assorted co-administered antibiotics. Therefore, experience with tigecycline in the treatment of critically ill patients, including patients with VAP, is lacking.
The aim of this retrospective study was to report our experience of the microbiological and clinical effectiveness of tigecycline against MDRAB infections in seriously ill patients, including those with VAP.
MATERIALS AND METHODS
Study design and data collection
A retrospective study was conducted at Gangnam Severance Hospital, Seoul, Korea. This study was approved by the Institutional Review Board, Gangnam Severance Hospital, Yonsei University College of Medicine (IRB Number: 3-2011-0017).
The enrolled patients were intensive care unit (ICU) admitted adults (age ≥18 years) with MDRAB infections who were treated with tigecycline between April 1, 2009 and March 31, 2010. Tigecycline was administered intravenously at a dose of 50 mg every 12 hours after a loading dose of 100 mg. Exclusion criteria were as follows: age <18 years, duration of tigecycline treatment <5 days, administration of tigecycline as empirical treatment or for infections involving organisms other than MDRAB. For polymicrobial infections, patients were included only if tigecycline treatment was determined by the presence of MDRAB.
Data recorded for each patient included the following: age, gender, associated co-morbidities and previous antimicrobial regimens, site of infection, major reason for hospital admission and length of intensive care unit stay. Disease severity was assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) scores upon ICU admission and on day 1 of tigecycline therapy.
Definitions
MDRAB was defined if A. baumannii isolate was resistant to representative antibiotics of at least three different classes of antimicrobial agents such as aminoglycosides, anti-pseudomonal penicillins, carbapenems, cephalosporins, quinolones, colistin, ampicillin/sulbactam and/or tetracyclines.20,21 We defined MDRAB infection as clinical findings of pneumonia such as fever, changing patterns of sputum or signs of SIRS and identification of MDRAB in the respiratory or blood samples with suspected pathogen by attending physicians.22-24 We defined management as tigecycline administration and followed up the patients until tigecycline administration was stopped, without regard to clinical outcome of success or failure.
Clinical outcomes were evaluated by attending physicians using data from patient files in cooperation with the investigators. The decisions to begin and end the tigecycline treatment were made by the attending physician. The duration of tigecycline treatment was decided according to its clinical responses.
Clinical response at the end of treatment was defined as success (complete or partial resolution of signs/symptoms) or failure (no improvement or deterioration of signs/symptoms of infection). Microbiological response was defined as success (eradication/sterile culture results during or after the course of antibiotic therapy) or failure (continuously positive culture results or the development of a superinfection due to treatment). In polymicrobial infections, microbiological response was considered positive if MDRAB was eradicated from the primary site of infection.
VAP, bloodstream infections (BSI), and sepsis were defined according to American Thoracic Society/Centers for Disease Control and Prevention clinical diagnostic criteria.25
All-cause mortality during 14 days from the start of tigecycline treatment was recorded. Death caused by primary infection was defined as death occurring without resolution of signs and symptoms of infection and with no other cause identified.
Nephrotoxicity was defined as an increase in serum creatinine level of more than 50% above baseline (recorded on the first day of tigecycline treatment) at a value above the upper normal limit (>1.3 mg/dL). Underlying chronic renal failure was defined by a baseline creatinine clearance rate of less than 60 mL/min/1.73 m2.
Statistical analysis
Data were analyzed with SPSS version 18.0 statistical software (SPSS, Chicago, IL, USA). All tests were two-sided. Categorical variables were compared by Fisher's exact test and Pearson's chi-square test as appropriate. Continuous variables were compared with Student's t-test. A p-value ≤0.05 was considered statistically significant.
RESULTS
Patient characteristics
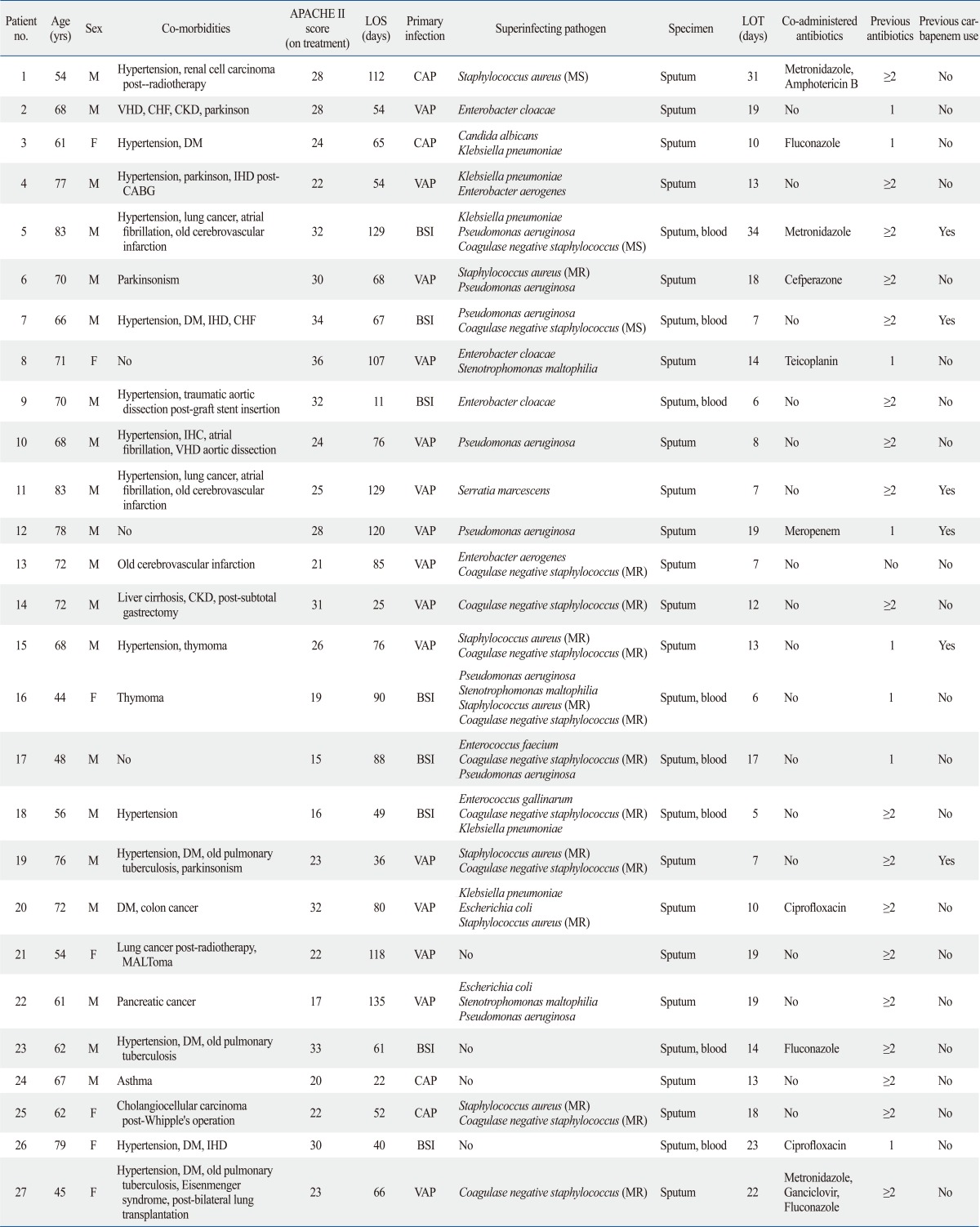
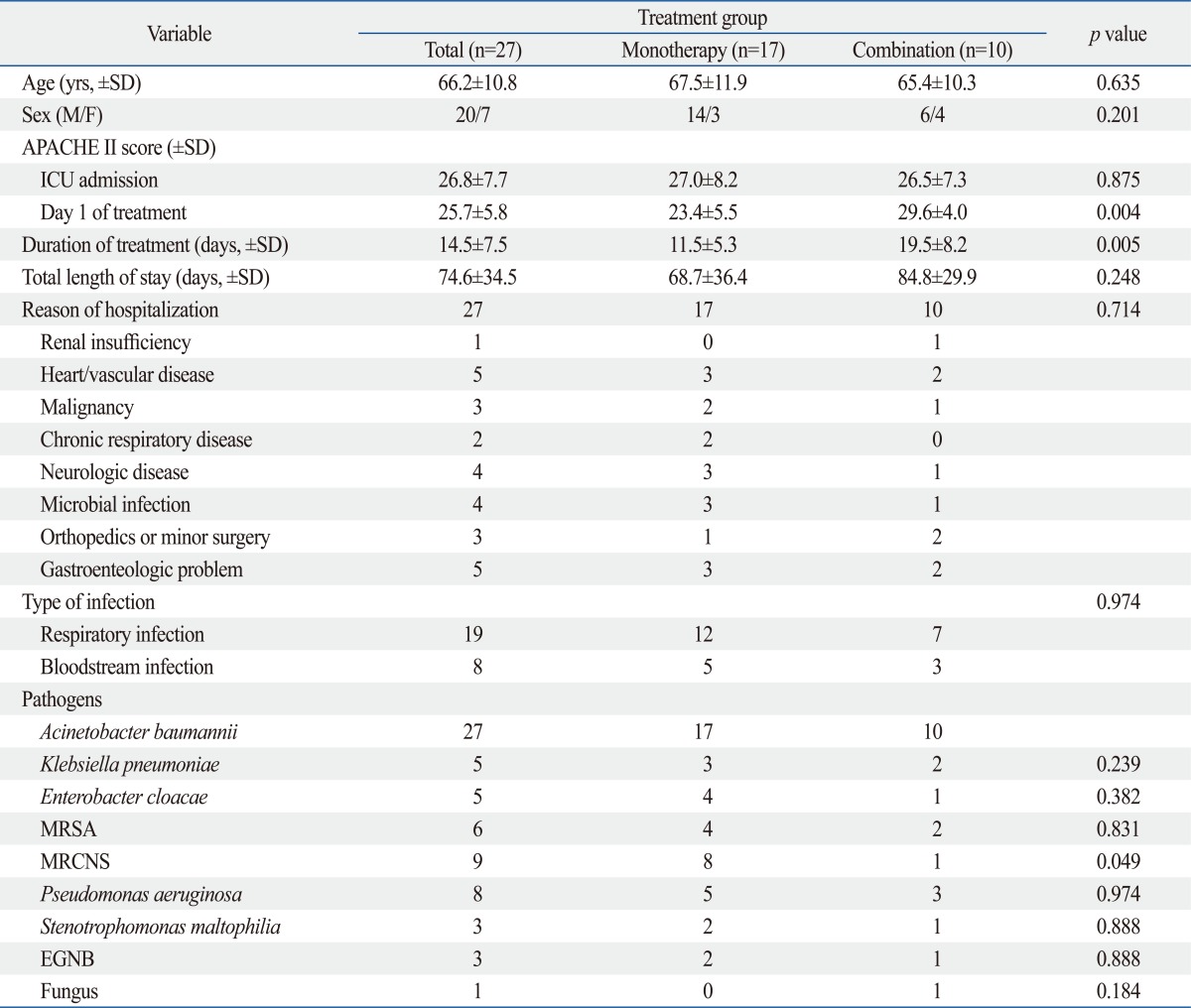
A total of 27 patients received tigecycline for MDRAB infections between April 1, 2009 and March 31, 2010. All patients were treated with the standard regimen of a 100 mg loading dose followed by 50 mg intravenous tigecycline every 12 hours. Tigecycline administration was started in ICU, but some patients were transferred to general ward during management while clinically improved. Patient and pathogen characteristics are recorded in Table 1. The mean patient age was 66.2±10.8 years (44-83 years), and 20 (74.1%) patients were male. The median APACHE II score upon ICU admission was 26.8±7.7 and 25.7±5.8 on day 1 of tigecycline treatment. The median length of stay was 74.6 days (range, 11-135) (Table 2).
Table 1.
Characteristics of Patients Treated with Tigecycline for Infections Involving MDRAB
no., number; APACHE II score, Acute Physiology and Chronic Health Evaluation II score; LOS, length of inpatient stay; LOT, length of treatment with tigecycline; DM, diabetes mellitus; M, male; F, female; IHD, ischemic heart disease; VHD, valvular heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; MS, methicillin sensitive; MR, methicillin resistant; CAP, community-acquired pneumonia; VAP, ventilator-associated pneumonia; BSI, bloodstream infection; MDRAB, multidrug-resistant Acinetobacter baumannii.
Table 2.
Characteristics of Patients Treated with Tigecycline
ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MRCNS, methicillin-resistant coagulase-negative Staphylococci.; EGNB, enteric gram-negative bacillus; SD, standard variation; M, male; F, female; APACHE II score, Acute Physiology and Chronic Health Evaluation II score.
Tigecycline was considered to be the drug of choice for MDRAB infection because 5 patients showed colistin-resistant MDRAB infection and 7 patients showed renal failure (eGFR <60 mL/min/BSA). Fifteen patients could not be treated with colistin due to the instability in stock of the drug in Korea at the time (data not shown).
Four patients were treated with tigecycline for MDRAB as a single pathogen and 23 patients were treated for polymicrobial infections. Methicillin-resistant coagulase-negative staphylococci (33.3%, 9/27) were the most common co-infected organisms, followed by Pseudomonas aeruginosa (29.6%, 8/27) and methicillin-resistant Staphylococcus aureus (MRSA) (22.2%, 6/27) (Table 1 and 2).
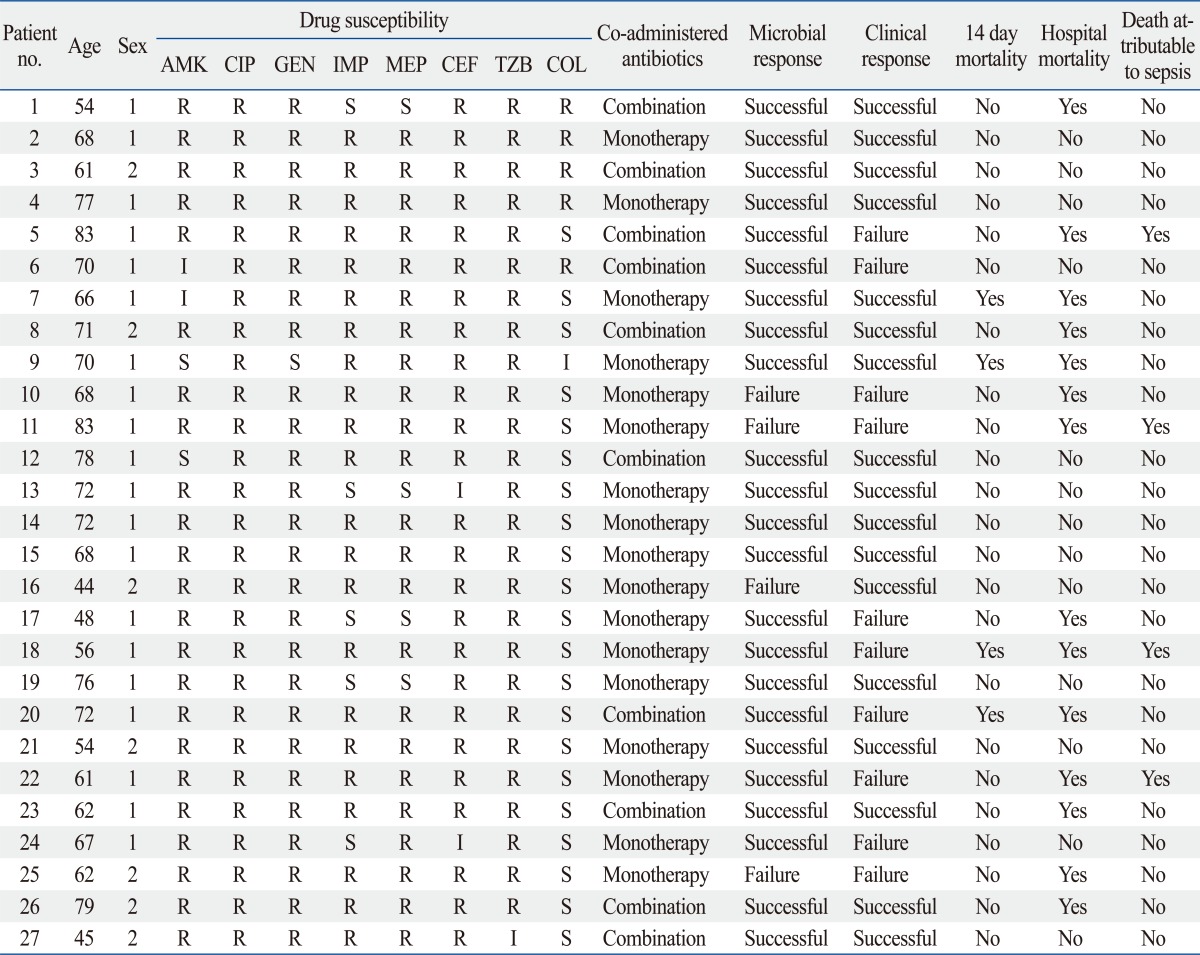
Drug susceptibility test revealed resistance to imipenem and meropenem in 85% (22/27) and 81% (23/27) of cases, respectively. Resistance to colistin was observed in 22.2% (6/27, 5 of resistance and 1 of intermediate) of cases (Table 3).
Table 3.
Clinical and Microbiological Outcomes of Patients Treated with Tigecycline
no., number; AMK, amikacin; CIP, ciprofloxacin; GEN, gentamicin; IMP, imipenem; MEP, meropenem; CEF, cefperazone; TZB, tazolactam/sulbactam; COL, colistin; R, resistance; I, intermediate; S, sensitive.
Twenty-six patients had been treated with antibiotics prior to the current hospitalization, and 18 of these patients (69.0%) were treated with more than two antibiotics. Piperacillin-tazobactam, respiratory quinolones, carbapenems, and third/fourth generation cephalosporins were the most frequently used prior antibiotics (37.0%, 33.3%, 25.9% and 25.9%, respectively) (data not shown). The average length of treatment was 14.5±7.5 days.
Tigecycline was administered in 17 patients (54.4%) as monotherapy and in combination with other antibiotics in 10 patients. In combination therapy group, 2 cases administered another antibiotics or antifungal agents for other pathogens prior to MDRAB identification. Metronidazole was the most common concomitant antibiotic (30.0%). It was not for MDRAB infection, but overlap maintenance for prior clinically suspected anaerobic infection. Three patients in the combination therapy group were treated with antifungal agents such as fluconazole and/or amphotericin B.
There were no significant differences in the APACHE II scores at ICU admission between the monotherapy group and the combination therapy group (27.0±8.2 vs. 26.5±7.3); however the APACHE II scores on day 1 of treatment (23.4±5.5 vs. 29.6±4.0) and the duration of treatment (11.5±5.3 vs. 19.5±8.2, days) were significantly different between the groups, suggesting that clinicians tend to choose combination therapy for clinically deteriorated patients (Table 1 and 2).
Median duration between ICU admission and beginning of treatment was 33.8±28.6 days, and there was no statistical difference between monotherapy group and combination group (37.1±33.7 vs. 27.9±16.0 days, p=0.453) (data not shown). Some patients were admitted to ICU for various reasons and infected with MDRAB during the ICU stay. Therefore, the duration between ICU admission and the beginning of tigecycline treatment for MDRAB infection did not show clinical significance.
Treatment outcomes
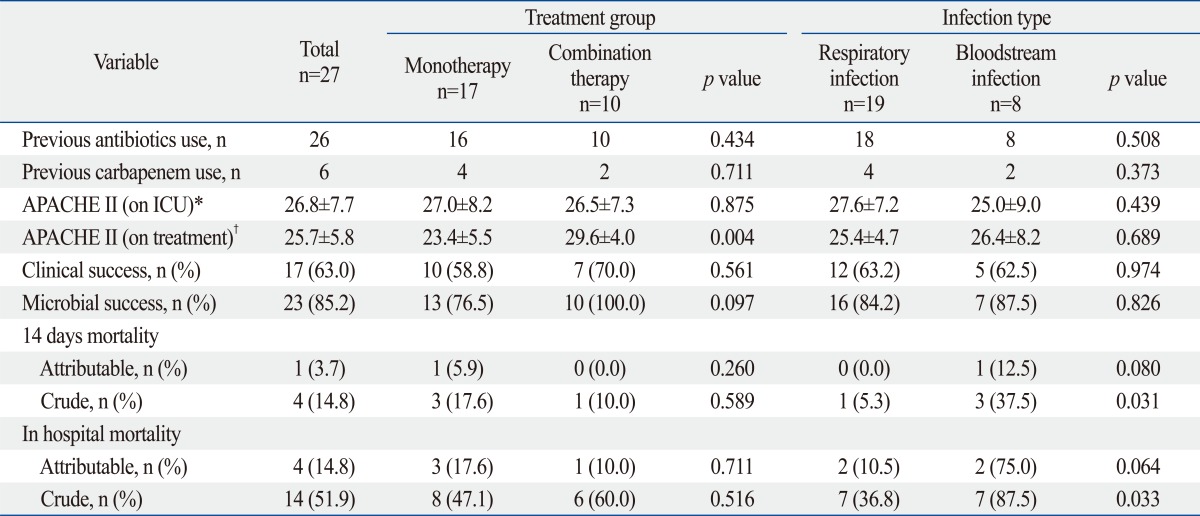
In 23 cases (85.2%), MDRAB was eradicated from the site of infection, however, only 17 cases (63.0%) showed positive clinical response. Four patients died within 14 days of initiating treatment, representing a crude mortality rate of 14.8%. One of these deaths was attributable to sepsis. An overall in-hospital mortality rate of 51.9% was observed, and death in four cases was attributable to sepsis (Table 4).
Table 4.
Clinical Outcome and Mortality in Patient Treated with Tigecycline
n, number of patient; ICU, intensive-care unit; APACHE II score, Acute Physiology and Chronic Health Evaluation II score.
*On ICU: APACHE II score on 1st day of intensive care unit admission.
†On treatment: APACHE II score on 1st day of treatment with tigecycline.
Subanalysis by treatment group and infection
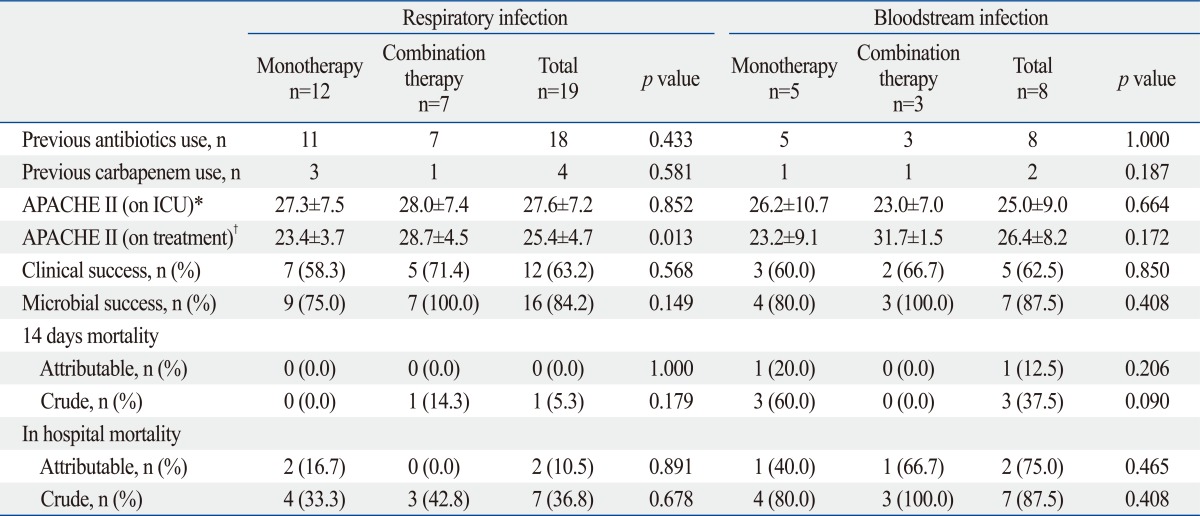
The combination therapy group showed better clinical and microbial success rates without statistical significance. For 14-day mortality and in-hospital mortality, there were no significant differences between the monotherapy and combination therapy groups. Clinical and microbial success rate were not affected by infection type (Table 4).
In respiratory infections, 4 of 19 patients were treated for community-acquired pneumonia and others were treated for VAP. Twelve of 19 patients received monotherapy with tigecycline and others received combination therapy including tigecycline. Overall, the successful clinical response rate was 63.2%, and the microbial response rate was 84.2%. Two patients with clinical failure died due to superinfection. In BSI, MDRAB was isolated from blood and sputum in all patients. Five of eight patients received monotherapy and others received combination therapy including tigecycline. The overall successful clinical response rate was 62.5%, and the microbial response rate was 87.5%. Two patients with clinical failure died due to superinfection (Table 5).
Table 5.
Subanalysis of Clinical Outcome and Mortality in Patient according to Infection Type
n, number of patient; ICU, intensive-care unit; APACHE II score, Acute Physiology and Chronic Health Evaluation II score.
*On ICU: APACHE II score on 1st day of intensive care unit admission.
†On treatment: APACHE II score on 1st day of treatment with tigecycline.
DISCUSSION
Tigecycline has been approved for the treatment of complicated intra-abdominal infections, complicated skin and soft tissue infections, and community-acquired bacterial pneumonia.26 However, the role of tigecycline in VAP is uncertain and it has been considered an optional treatment for VAP caused by MDR organisms, except for Pseudomonas aeruginosa.
Accumulated evidence has identified that tigecycline has considerable microbiological activity against MDRAB, including carbapenem-resistant Acinetobacter spp. Nevertheless, many studies have revealed that the activity of tigecycline is not optimal, suggesting that the use of tigecycline may not comprise a definitive solution of the growing problem of MDRAB. Nonetheless, the utility of tigecycline should not be disregarded because other antimicrobial agents, except polymyxins, are not reliably active against carbapenem-resistant A. baumannii isolates.27 Recently, the Food and Drug Administration (FDA) determined that tigecycline resulted in increased mortality risk, especially in hospital-acquired pneumonia, compared with other antibiotics. We studied only tigecycline-treated patients infected with MDRAB, but not patients with VAP in general.
We examined the microbiological activity and clinical effectiveness of tigecycline against MDRAB infections in 31 seriously ill patients, including patients with VAP, and observed a weak correlation between microbiological clearance and clinical outcome. Overall, only 63.0% had positive clinical outcomes, whereas 85.2% had microbial eradication. A recent systematic review27 found an overall response rate of 76% to tigecycline for a wide range of MDRAB infections. Several studies on the treatment of VAP have been reported; the proportion of global clinical success is 69-88%28-30 and microbiological eradication is 80%28 with or without concomitant antibiotic therapy. The lack of correlation between clinical and microbiological outcomes increases the debate regarding the inherent pathogenicity of A. baumannii.
In respiratory infection, the clinical success rate was only 63.2% with or without concomitant antibiotics (58.3% vs. 71.4%), despite the fact that 84.2% showed microbial eradication. Interestingly, in the BSI group the microbial eradication rate was 87.5% and the clinical success rate was 62.5% regardless of concomitant antibiotic treatment (60.0% vs. 66.7%). We, therefore, hypothesize that tigecycline monotherapy was ineffective in several patients because most clinical failures were due to superinfection and there was a high Pseudomonas aeruginosa infection rate (29.6%). Although multidrug resistant Pseudomonas aeruginosa was expected to be the most important and common pathogen in superinfections, we found that patients with superinfections tended to have lower previous or concomitant use of carbapenems and colistin. In addition, probable pharmacokinetic concerns might be related to the failure to improve infections caused by sensitive microorganisms. Acquired resistance to tigecycline and superinfections, due to intrinsically resistant pathogens to tigecycline, supports the possible benefits from combination therapy, as hypothesized in large prospective and randomized studies.30 Meanwhile, selection of patients to receive tigecycline remains important.
Above all, most of the respiratory infections (15/19) and BSI (7/8) represented hospital acquired pneumonia including VAP, and we cannot at present affirm the efficacy of tigecycline for VAP. Nevertheless, randomized controlled trials should provide more concrete evidence. Randomized phase III trial to compare tigecycline and imipenem/cilastatin for nosocomial pneumonia25 found that clinical cure rates are poorer for tigecycline than with imipenem/cilastatin in the subset of VAP patients, therefore, tigecycline has not been approved for the treatment of VAP, and clinicians have considered tigecycline to be a useful option for the treatment of patients with VAP based on its good in vitro activity and a pharmacokinetic profile with high intrapulmonary concentrations.17,20 In fact, the excellent antimicrobial activity of tigecycline may be evidenced by the relatively long post-antibiotic effect of up to 3 hours against A. baumannii, even though pharmacokinetic and pharmacodynamic data indicate that blood concentrations are suboptimal for maximal antibacterial activity.31
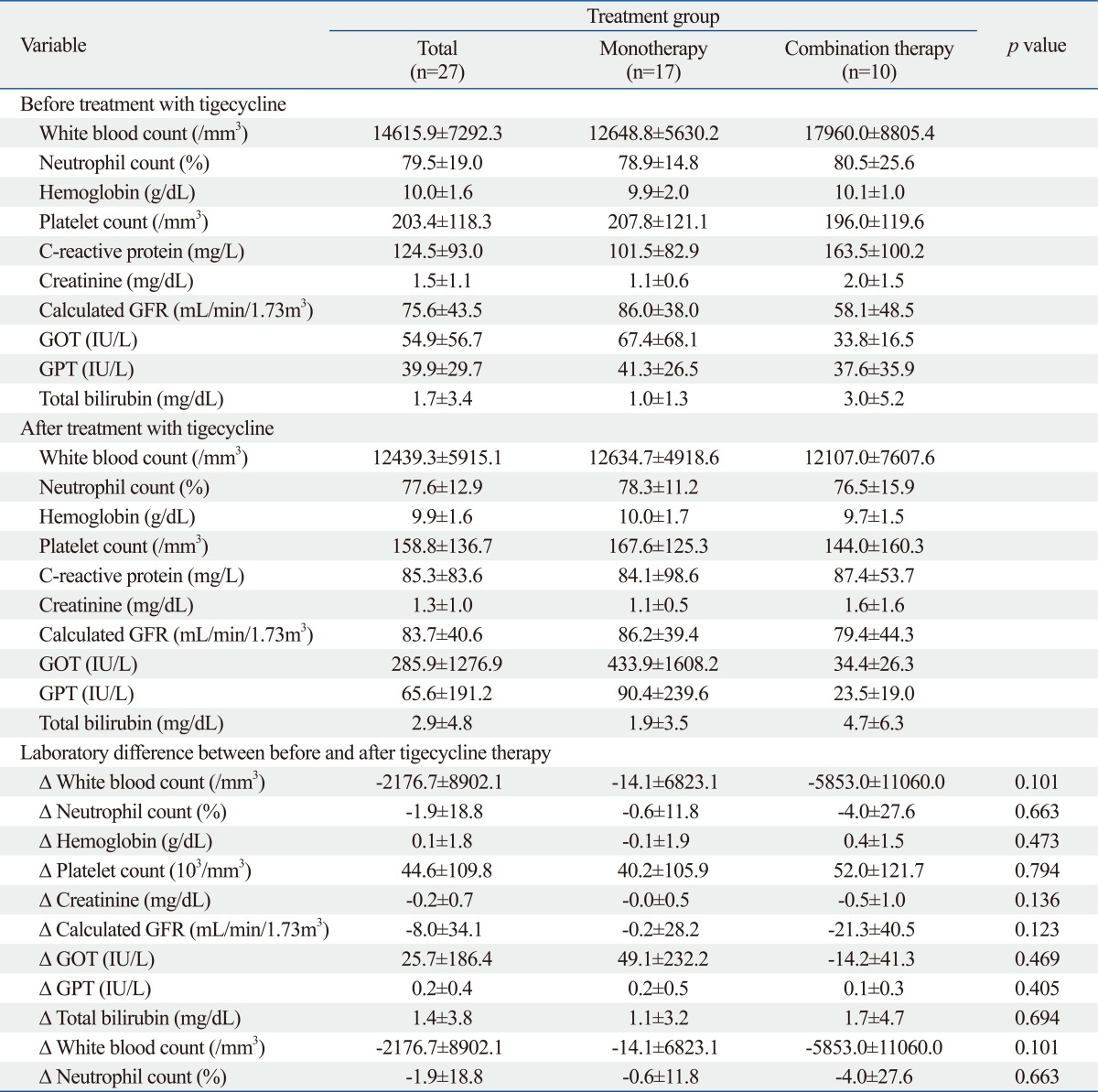
There were no differences in laboratory findings including GOT/GPT, total bilirubin, renal function, hemoglobin, or platelet count between the monotherapy group and the combination therapy group (Supplementary Table 1). There were also no differences in symptoms such as gastrointestinal problems including pseudomembraneous colitis and skin rashes (data not shown). We, therefore, suggest that concomitant antibiotic use may not significantly increase drug toxicity. In our study, newly onset nephrotoxicity in patients with normal renal function or renal function decline in patients with renal insufficiency was not observed (patient No. 1, 2, 3, 5, 7, 8, 9, 10, 11, 20, 26). Considering the 10% incidence of nephrotoxicity of colistin, this result suggests that tigecycline is an important option for patients with chronic renal failure.32
Supplementary Table 1.
Laboratory Findings before and after Treatment with Tigecycline
GFR, glomerular filtration rate; GOT, glutamyl oxloacetic transaminase; GPT, glutamyl pyruvic transaminase.
Δ: laboratory difference between before tigecycline therapy and after tigecycline therapy.
Our study has several limitations. First, our study employed a retrospective design and small number of patients at a single tertiary hospital, therefore, generalization to other clinical setting is limited. Second, there is a lack of pharmacokinetic and pharmacodynamic data, as we were unable to measure serum and intrapulmonary levels of tigecycline.
In conclusion, we examined the clinical and microbial efficacy of tigecycline for MDRAB infection including the isolates that were resistant to carbapenem and/or colistin, and observed a relatively low clinical success rate although the microbial eradication rate was high, probably due to superinfections in VAP and bacteremia. Although there was no significant difference between monotherapy and combination therapy groups, the combination therapy group showed better clinical and microbial success rates. We attribute the benefits from combination therapy to acquired resistance to tigecycline and superinfections with intrinsically resistant pathogens to tigecycline. Regarding the lack of data on tigecycline in the treatment of critically ill patients with MDRAB infection, we suggest that clinicians should limit tigecycline monotherapy for MDRAB infection to critically ill patients and consider the combination with anti-pseudomonal agent, while making reference to drug susceptibility test, until large controlled clinical trials should be conducted.
ACKNOWLEDGEMENTS
This study was supported by the Institutional Grant from Yonsei University College of Medicine (6-2007-0100) to the Human Barrier Research Institute. The authors are grateful for statistical analyses provided by medical research supporting section of Yonsei University College of Medicine.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wareham DW, Bean DC, Khanna P, Hennessy EM, Krahe D, Ely A, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. 2008;27:607–612. doi: 10.1007/s10096-008-0473-y. [DOI] [PubMed] [Google Scholar]
- 3.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 4.Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falagas ME, Kasiakou SK, Rafailidis PI, Zouglakis G, Morfou P. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother. 2006;57:1251–1254. doi: 10.1093/jac/dkl130. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cisneros JM, Reyes MJ, Pachón J, Becerril B, Caballero FJ, García-Garmendía JL, et al. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 9.Humphreys H, Towner KJ. Impact of Acinetobacter spp. in intensive care units in Great Britain and Ireland. J Hosp Infect. 1997;37:281–286. doi: 10.1016/s0195-6701(97)90144-4. [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011;52:879–891. doi: 10.3349/ymj.2011.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalla-Costa LM, Coelho JM, Souza HA, Castro ME, Stier CJ, Bragagnolo KL, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. J Clin Microbiol. 2003;41:3403–3406. doi: 10.1128/JCM.41.7.3403-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuel RJ, Shin GY, Farrag N, Holliman R. Endemic carbapenem-resistant Acinetobacter baumannii in a London hospital. J Antimicrob Chemother. 2003;52:141–142. doi: 10.1093/jac/dkg276. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, et al. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011;52:793–802. doi: 10.3349/ymj.2011.52.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin BS, Han SH, Choi SH, Lee HS, Jeong SJ, Choi HK, et al. The characteristics of metallo-β-lactamase-producing gram-negative bacilli isolated from sputum and urine: a single center experience in Korea. Yonsei Med J. 2011;52:351–357. doi: 10.3349/ymj.2011.52.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coelho JM, Turton JF, Kaufmann ME, Glover J, Woodford N, Warner M, et al. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J Clin Microbiol. 2006;44:3623–3627. doi: 10.1128/JCM.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner PJ. MYSTIC Europe 2007: activity of meropenem and other broad-spectrum agents against nosocomial isolates. Diagn Microbiol Infect Dis. 2009;63:217–222. doi: 10.1016/j.diagmicrobio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Rhomberg PR, Jones RN. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999-2008) Diagn Microbiol Infect Dis. 2009;65:414–426. doi: 10.1016/j.diagmicrobio.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NC, Wareham DW. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother. 2009;63:775–780. doi: 10.1093/jac/dkn555. [DOI] [PubMed] [Google Scholar]
- 19.Dizbay M, Altuncekic A, Sezer BE, Ozdemir K, Arman D. Colistin and tigecycline susceptibility among multidrug-resistant Acinetobacter baumannii isolated from ventilator-associated pneumonia. Int J Antimicrob Agents. 2008;32:29–32. doi: 10.1016/j.ijantimicag.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Oh JY, Kim KS, Jeong YW, Cho JW, Park JC, Lee JC. Epidemiological typing and prevalence of integrons in multiresistant Acinetobacter strains. APMIS. 2002;110:247–252. doi: 10.1034/j.1600-0463.2002.100307.x. [DOI] [PubMed] [Google Scholar]
- 21.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(Pt 12):1619–1629. doi: 10.1099/jmm.0.46747-0. [DOI] [PubMed] [Google Scholar]
- 22.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 23.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 25.Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68:140–151. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56:470–480. doi: 10.1093/jac/dki248. [DOI] [PubMed] [Google Scholar]
- 27.Karageorgopoulos DE, Kelesidis T, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J Antimicrob Chemother. 2008;62:45–55. doi: 10.1093/jac/dkn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer JJ, Goff DA, Stevenson KB, Mangino JE. Early experience with tigecycline for ventilator-associated pneumonia and bacteremia caused by multidrug-resistant Acinetobacter baumannii. Pharmacotherapy. 2007;27:980–987. doi: 10.1592/phco.27.7.980. [DOI] [PubMed] [Google Scholar]
- 29.Curcio D, Fernández F, Vergara J, Vazquez W, Luna CM. Late onset ventilator-associated pneumonia due to multidrug-resistant Acinetobacter spp.: experience with tigecycline. J Chemother. 2009;21:58–62. doi: 10.1179/joc.2009.21.1.58. [DOI] [PubMed] [Google Scholar]
- 30.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58:273–284. doi: 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Jamal W, Salama M, Dehrab N, Al Hashem G, Shahin M, Rotimi VO. Role of tigecycline in the control of a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect. 2009;72:234–242. doi: 10.1016/j.jhin.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]