Figure 1.
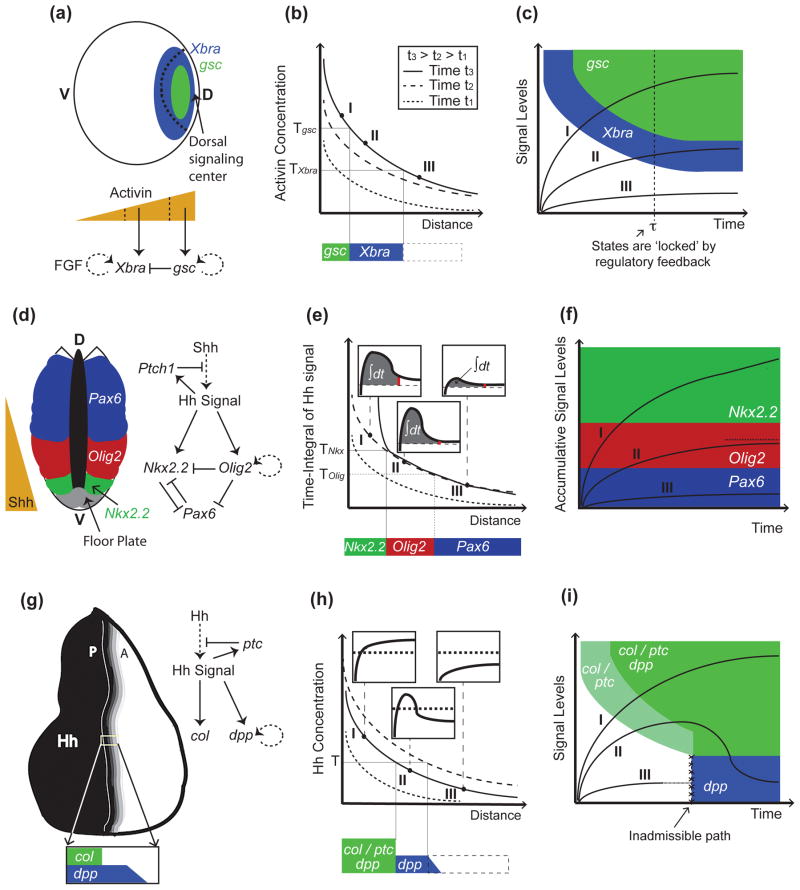
Models of morphogen gradient interpretation. (a-c), Mesoderm specification in Xenopus depends on activin concentrations. Activin is secreted from the dorsal signaling center, and forms a dorsal (D) to ventral (V) gradient that activates Xbra and gsc at successively increasing concentration thresholds (Tgsc>TXbra; (a)). In (b), (as well as in (e), and (h)), three gradients at successive time points are shown indicating the dynamics displayed by the gradients (legend only displayed in (b), but also applies to (e) and (h)). In the case of activin, genes are activated sequentially; cells exposed to high activin concentrations express first Xbra and then gsc (trajectory I in (c)). Then, Xbra is turned off by Gsc (a), resulting in mutually-exclusive domains (a). Once cells have acquired a stable pattern (e.g., after time τ in the trajectories in (c)), they no longer require activin signaling and are no longer under the control of the morphogen thresholds (dotted line in (c)). Cell states are now irreversible and maintained by positive feedback loops downstream of activin signaling ((a); dotted lines in the regulatory networks denote that the interaction may be not direct). (d-g), In the developing spinal cord, Shh is secreted in the floor plate in the ventral-most portion of the neural tube and establishes the expression of Nkx2.2, Olig2, and Pax6, among other neural markers, in specific domains of the neural tube (d). Not shown in (d-f), for the sake of simplicity, is that low levels of Pax6 persist in the Olig2 domain. As in the case of activin, the neural progenitor markers Olig2 and Nkx2.2 are also established sequentially (trajectory I in (f)), but in this case, the order of activation depends on the integration of Hh signaling over time (e). This is accomplished by a feed-forward loop in which activation of Nkx2.2 requires both sustained Shh exposure and Olig2-mediated repression of the Pax6 inhibitor (d). In (e), the time-integral (area under signal vs. time curves; see insets) is higher closer to the Shh source, because desensitization of cells is slower when exposed to higher Shh concentrations. In this model, cell fates are determined by thresholds in accumulated signal levels (f). In (f), it is assumed that the time-integral of the signal is bounded, at least in cells marked by Olig2 (dotted line in (f)), although it is unclear how this is accomplished. (g-i) In the Drosophila wing disc, Hh is expressed in the posterior (P) compartment and establishes a short-range gradient into the anterior (A) compartment (g). col (and ptc) and dpp become expressed in different domains as a consequence to a refinement in the Hh gradient (h). This ‘shift’ results from increased Hh sequestration and destruction by Ptc as ptc becomes upregulated by Hh signaling (g). Thus, unlike cells exposed to sustained signaling (I), or not receiving Hh at all (III), some cells in the middle of the gradient (II) receive Hh signaling only transiently (insets in (h)). This model of signal interpretation (represented by the state-space in (i)) only requires a single switching threshold, T. Unlike in the previous examples (panels (c) and (f)), col/ptc are turned on before dpp (i), despite the fact that cells are exposed to increasing concentrations. In (i), the white and blue domains are not connected (i.e., trajectory I is assumed to reach the white-blue boundary in infinite time).