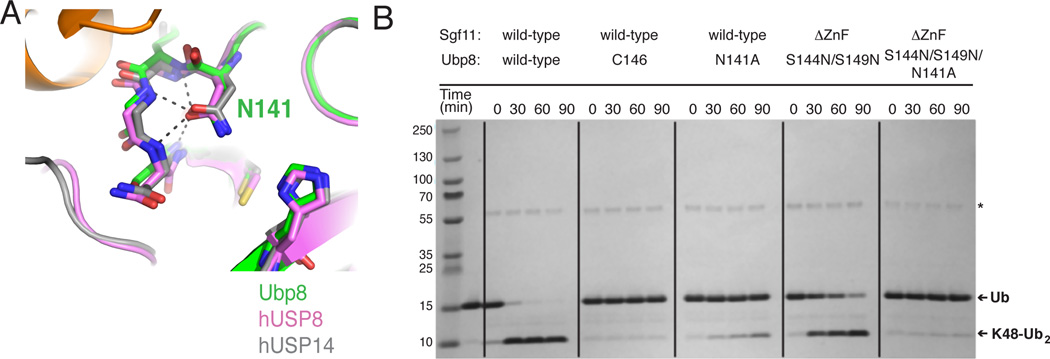
Figure 6. Ubp8-N141 is important for DUBm catalysis.
(A) Superpositions showing hydrogen bonding of conserved oxyanion hole residue (N141 in Ubp8) to backbone amides. Apo-Ubp8 (2MHH, gray), hUSP8 (2GFO, pink), and hUSP14 (2AYN, gray).
(B) Effect of Ubp8 active site mutations on DUBm activity Gel shows time course for cleavage of K48-linked diubiquitin for the intact DUBm-WT, DUBm-Ubp8C146A, DUBm-Ubp8N141A, DUBm-ΔSgf11-ZnF-Ubp8S144N/S149N, and DUBm-ΔSgf11-ZnF-Ubp8S144N/S149N/N141A.