TABLE 2.
Structures of Pyridoxal-6-azoaryl-5-phosphate Derivatives Examined as Antagonists at P2 Receptors
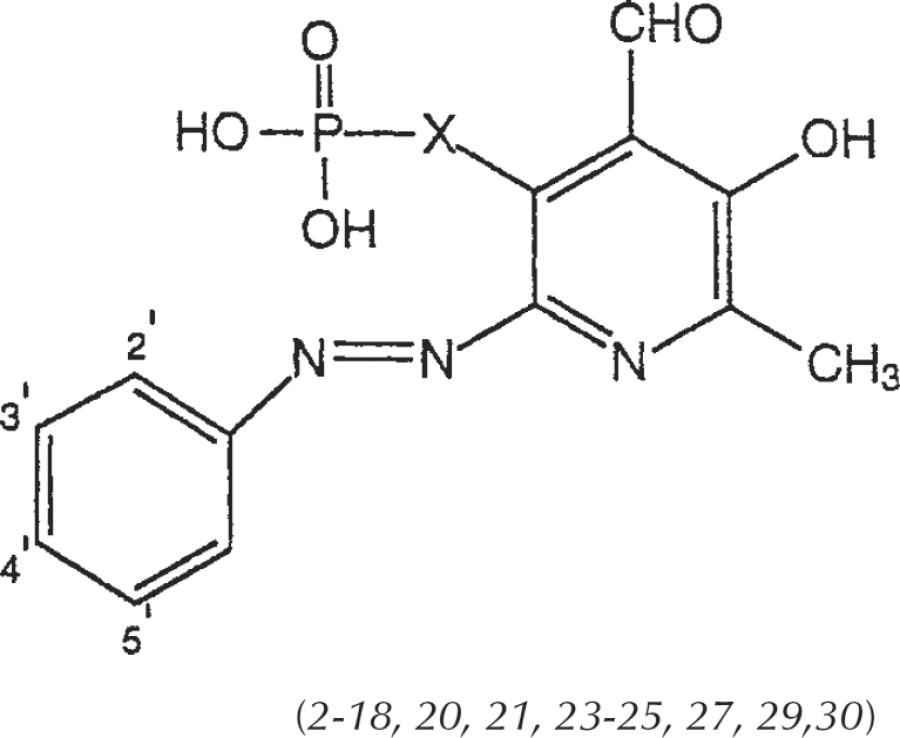
| |||||
|---|---|---|---|---|---|
|
| |||||
| Compounda | 2′ | 3′ | 4′ | 5′ | X |
| 2 | H | H | H | H | CH2O |
| 3 | SO3H | H | H | H | CH2O |
| 4 | H | SO3H | H | H | CH2O |
| 5 | H | H | SO3H | H | CH2O |
| 6 (PPADS) | SO3H | H | SO3H | H | CH2O |
| 7 (IsoPPADS) | SO3H | H | H | SO3H | CH2O |
| 8 | OCH3 | H | H | SO3H | CH2O |
| 9 | H | COOH | H | H | CH2O |
| 10 | H | H | COOH | H | CH2O |
| 11 | COOH | Cl | H | H | CH2O |
| 12 | Cl | H | H | H | CH2O |
| 13 | Cl | H | H | SO3H | CH2O |
| 14 | Cl | H | H | COOH | CH2O |
| 15 | H | Cl | COOH | H | CH2O |
| 16 | Cl | H | H | NO2 | CH2O |
| 17 | NO2 | H | H | NO2 | CH2O |
| 18 | H | H | NO2 | H | CH2O |
| 20 | SO3H | H | H | SO3H | CH2 |
| 21 | Cl | H | H | SO3H | CH2 |
| 23 | SO3H | H | H | SO3H | CH2CH2 |
| 24 | Cl | H | H | SO3H | CH2CH2 |
| 25 | H | H | COOH | H | CH2CH2 |
| 27 | SO3H | H | H | SO3H | CH=CH |
| 29 | SO3H | H | H | SO3H | CH2CH2CH2 |
| 30 | Cl | H | H | SO3H | CH2CH2CH2 |
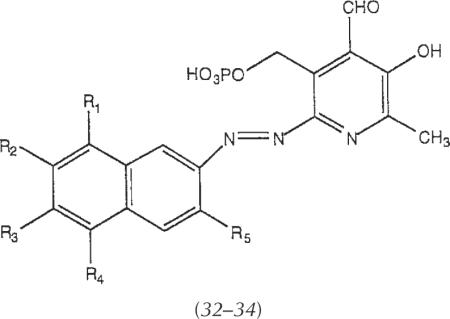
| |||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| 32 | H | SO3H | H | SO3H | H |
| 33 | SO3H | H | SO3H | H | H |
| 34 | H | SO3H | H | SO3H | SO3H |
Compounds 1 (X=CH2O), 19 (X=CH2), 22 (X=CH2CH2), 26 (X=CH=CH), and 28 (X=CH2CH2CH2), and 31 (X=CH=CHCH2) are non–azo-linked precursors (see Schemes 1 and 2). Compounds 1, 2, 3, 5, 6, 17, and 18 were reported by Lambrecht etal., 1996.
