Abstract
The objective of this article is to study the outcome after translabyrinthine surgery for vestibular schwannomas, with special focus on the facial nerve function. The study design is a case series from a national centralized database and it is set in two University Hospitals in Denmark. Participants were 1244 patients who underwent translabyrinthine surgery during a period of 33 years from 1976 to 2009. Main outcome measures were tumor removal, intraoperative facial nerve preservation, complications, and postoperative facial nerve function. In 84% patients, the tumor was totally resected and in ~85% the nerve was intact during surgery. During 33 years, 12 patients died from complications to surgery and ~14% had cerebrospinal fluid leakage. Before surgery, 74 patients had facial paresis and 46% of these improved after surgery. In patients with normal facial function, overall ~70% had a good outcome (House-Brackmann grade 1 or 2). The chance of a good outcome was related to tumor size with a higher the chance the smaller the tumor, but not to the degree of tumor removal. In ~78% of the patients with facial paresis at discharge the paresis improved over time, in ~42% from a poor to a good function. The translabyrinthine approach is generally efficient in tumor control and with satisfactory facial nerve outcome. With larger tumors the risk of a poor outcome is evident and more data on patients managed with alternative strategies are warranted.
Keywords: vestibular schwannoma, translabyrinthine surgery, outcome, facial nerve
The optimal treatment of patients with vestibular schwannomas (VS) is still debated. Watchful waiting with serial magnetic resonance imaging (MRI) is often recommended for elderly patients with smaller tumors, but in patients with larger tumors or in younger patients with established tumor growth some form of treatment is generally advocated.1,2 Surgery has traditionally been the treatment of choice for most patients, though recent studies have suggested stereotactic radiosurgery as an alternative treatment in selected patients with smaller tumors.3,4 The aim of surgery for VS is maximal tumor removal without complications, and relevant clinical endpoints are mortality, tumor control, facial function, hearing preservation, and postoperative cerebrospinal fluid (CSF) leakage. The probability of complications and side effects will depend on the surgical approach, basically through middle fossa, suboccipital/retrosigmoid, or translabyrinthine exposure.1,5,6
The incidence of VS in Denmark (5.2 million inhabitants) is ~19 per million per year and the treatment is centralized.1,7,8 Until 2010, the surgery was performed in the ENT department at the Gentofte University Hospital or in the University Clinic of Neurosurgery at the University Hospital of Copenhagen depending on tumor size (cut-off 25 mm extrameatal diameter). Today all surgeries are performed at the University Hospital of Copenhagen but still by both ENT and neurosurgeons (same cut-off limit). Since the centralization of the treatment in 1976, most surgeries have been through a translabyrinthine approach because of the excellent exposure of the facial nerve, the minimal cerebellar retraction, and the relatively few problems with CSF leakage1,7,9,10,11,12. Presently, all patients with smaller tumors are considered for hearing preserving surgery if the preoperative hearing is good.1,2,13 Here, we present the outcome of 1244 patients, who underwent translabyrinthine surgery for VS from the introduction of the procedure in 1976 until December 2009, which is the largest series published so far. We focus mainly on postoperative facial nerve function, since this is of major concern in this group of patients.
Material and Methods
Data from all Danish patients with VS are prospectively entered into a centralized clinical database. In this study, we retrospectively retrieved data from all patients who entered the database since the introduction of the translabyrinthine approach in 1976 until 31 December 2009 to allow for at least 1 year of follow-up after surgery. Overall, 2441 patients were registered in the database. Off these 1023 were followed by a “wait and scan” policy, 64 patients received radiotherapy as their primary treatment and were therefore excluded from the study, and 1354 underwent surgery as their primary treatment. Data from patients with neurofibromatosis type 2 were excluded as were data from patients who were operated through a middle fossa or retrosigmoid approach leaving 1244 patients for analysis. Data on preoperative symptoms other than hearing level and facial nerve function are not collected in the database. From 1985 MRI became available in Denmark and an increasing number of patients were diagnosed by this modality. From 1995 all patients had preoperative MRI except a few cases with extreme obesity or claustrophobia. According to international recommendations, tumors were categorized as intrameatal or intra- and extrameatal based on the preoperative scans, and the size of the tumors with extrameatal extention was recorded as the extrameatal diameter not including the part of the tumor in the internal auditory canal. Tumor size was then categorized into five groups (0 to 10 mm, 11 to 20 mm, 21 to 30 mm, 31 to 40 mm and > 40 mm).14 The facial nerve function was categorized into six groups (1 to 6) using the House-Brackmann (HB) grading scale,15 and postoperatively further split into good (Grade 1 and 2) and poor outcome (Grade 3 to 6). Preoperative audiograms were available from 984 patients (79.1%). Intraoperative estimates of the extent of tumor removal were described as complete, near total (less than 5% of original tumor size), or subtotal (>5% of original tumor size)14 and data were available from 1216 patients (97.8%). The facial nerve integrity was intraoperatively estimated by the surgeon as intact, thin or disrupted and data were available from 1219 (98.0%) patients. Data on facial function after surgery at the time of discharge from the hospital were available from 1217 patients (97.8%) and 1-year follow-up was available from 1209 patients (97.2%). Regrettably, the database does not include standardized information on the postoperative function of the other relevant cranial nerves, wherefore these aspects will not be covered in this report. Data on other variables were available from all patients. Descriptive statistics were calculated for all variables and presented as frequencies and percentages for categorical data and means and standard deviations or medians and ranges for continuous data. Tables were analyzed by χ2 statistics. In some calculations, the patients with intrameatal tumors were not included as the number of patients was small and the patients considered a separate entity. Significance was accepted at the 0.05 level. Data analysis was performed using SPSS 18 (IBM, New York, NY).
Results
Patient Characteristics
Data on demographics, tumor size, and preoperative hearing level and facial function are presented in Table 1.
Table 1. Patient Characteristics, N = 1244.
| Mean Age, Years (SD) | 53 (13) |
| Sex, n (%) | |
| Women | 691 (55.5) |
| Men | 553 (45.5) |
| Side, n (%) | |
| Right | 618 (49.5) |
| Left | 628 (50.5) |
| Tumor location, n (%) | |
| Intrameatal | 13 (1.0) |
| Intra- and extrameatal | 1231 (99.0) |
| Mean tumor size, mma (SD) | 25 (13) |
| Tumor size group, n (%)a | |
| 0–10 mm | 155 (12.6) |
| 11–20 mm | 373 (30.3) |
| 21–30 mm | 360 (29.2) |
| 31–40 mm | 172 (14.0) |
| >40 mm | 171 (13.9) |
| Mean preoperative hearing level (SD) | |
| Pure tone average, dB | 59 (25) |
| Discrimination loss, % | 50 (36) |
| Preoperative HB grade, n (%) | |
| 1 | 1170 (94.1) |
| 2 | 6 (0.5) |
| 3 | 63 (5.1) |
| 4 | 0 |
| 5 | 1 (0.1) |
| 6 | 4 (0.3) |
Tumors with extrameatal extention.
HB, House-Brackmann; SD, standard deviation.
Tumor Removal
In tumors with extrameatal extention, 1011 (84.0%) was totally resected, 165 (13.7%) were near totally resected, and 27 (2.2%) were subtotally resected to preserve facial nerve integrity and/or avoid other potential neurological deficits. The extent of tumor removal was statistically significant related to the size group of the tumors with a smaller chance of total tumor removal in the larger tumor groups (N = 1203, χ2 = 51.343, p < 0.0001).
Intraoperative Facial Nerve Preservation
In tumors with extrameatal extention, the facial nerve was intraoperatively estimated as intact in 1229 (85.2%) patients, as thin in 101 (8.4%) patients, and as disrupted in 78 (6.5%) patients. The chance of preserving an anatomical intact facial nerve decreased with increasing tumor size group ranging from 96.1% in the 0 to 10 mm group to 75.4% in the >40 mm group (N = 1208, χ2 = 57.383, p < 0.0001).
Complications
In the 33-year-studied time period 12 patients (1.0%) died within 1 month after surgery. Off these, eight died from the effects of postoperative hematomas, two from a pulmonary embolus, and one from a thrombosis of the basilar artery. The median size of the tumors of the 12 patients who died was 35 mm (range: 20 to 75 mm) and 4 had giant tumors with a diameter >35 mm. The patients who died were generally older than the overall population with a median age of 65 years (range: 30 to 75 years), and 5 were older than 70 years at the time of surgery. Another eight patients died within 1 year after the surgery. One died 36 days after surgery from Halothane hepatitis and/or sepsis, one died 44 days after surgery from sustained pneumonia, one died 54 days after surgery from the effect of a postoperative hematoma, and five died from reasons not related to the operation.
Out of the 1244 patients, 112 (9.0%) had CSF leakage from the surgical wound and 56 (4.5%) had postoperative rhinoliquorrhea. Of the 112 patients with wound leaks, 44 (39.3%) were treated with a pressure dressing or extra wound suturing, 24 (21.4%) had lumbar drainage, and 44 (39.0%) required revision surgery. Of the 56 patients with rhinoliquorrhea, 8 (14.3%) were treated conservatively, 21 (37.5%) with lumbar drainage and 27 (48.1%) required revision surgery. The risk of CSF leakage (wound or nose) significantly, but nonlinearly, depended on tumor size group with a risk as low as 8.0% in the 0 to 10 mm group, and as high as 25.5% in the 31 to 41 mm group (N = 1194, χ2 = 29.186, p < 0.0001).
Postoperative Facial Nerve Function
Before surgery, 74 patients had some degree of facial paresis (Table 1). Of these, 34 (46.0%) improved after surgery, in 31 (41.9%) cases from a poor facial function (HB 3 to 6) to a good function (HB 1 or 2). In 9 patients (12.2%) the paresis was unaffected by surgery, and in 29 patients (39.2%) the paresis deteriorated. Finally, two of these patients died within 1 year of surgery.
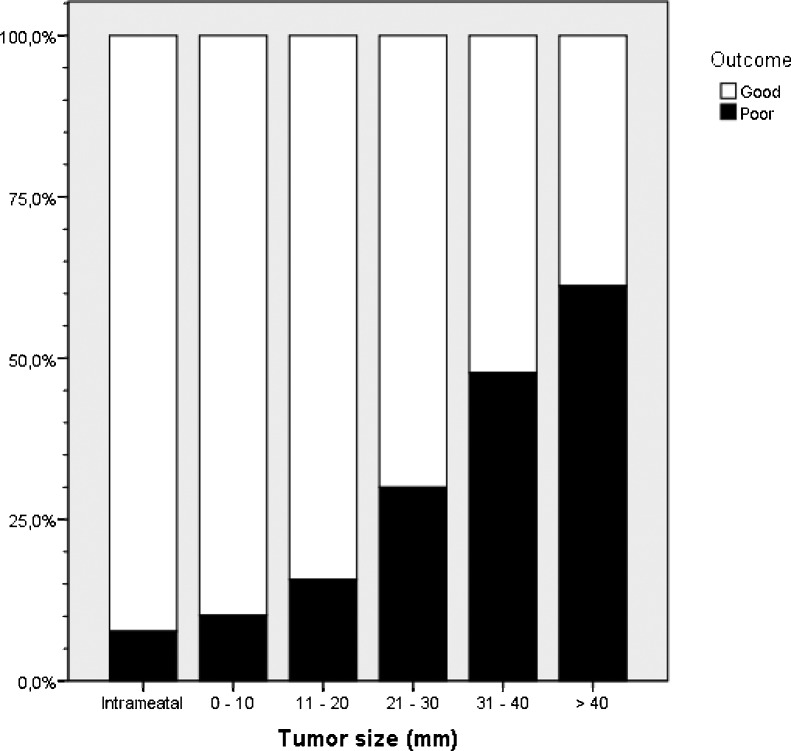
The 1-year facial function of the patients with normal preoperative function is shown in Table 2 and Fig. 1. Overall, out of 1152 patients, 810 (70.3%) had a good outcome (HB 1 or 2). In intrameatal tumors, 12 out of 13 (92.3%) had a good outcome, and in smaller extrameatal tumors (<25 mm) 538 out of 656 (82.0%) patients had a good outcome versus 260 out of 468 (55.6%) of the patients with tumors larger than 25 mm (N = 1137). For the patients with extrameatal tumors, the chance of a good clinical outcome was statistically significant related to tumor size group with a higher the chance the smaller the tumor (N = 1124, χ2 = 217.967, p < 0.0001). The chance of a good outcome was, however, not related to the degree of tumor removal (N = 1107, χ2 = 4.339, p = 0,114). Out of 960 patients with the facial nerve described as intact during surgery, 763 (79.5%) had a good facial function after 1 year, whereas only 30 out of 91 (33%) patients with the facial nerve described as thin during surgery had a good outcome. All patients with disrupted nerves had a poor facial nerve outcome.
Table 2. Facial Nerve Function After 1 Year Based on Tumor Size.
| Intrameatal | Intra- and Extrameatal | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–10 mm | 11–20 mm | 21–30 mm | 31–40 mm | >40 mm | ||||
| House- Brackmann grade | 1 | 12 (92.3%) | 116 (77.9%) | 246 (67.8%) | 166 (49.4%) | 54 (34.4%) | 44 (32.8%) | 638 (55.4%) |
| 2 | 17 (11.4%) | 55 (15.2%) | 66 (19.3%) | 27 (17.2%) | 8 (6.0%) | 172 (14.9%) | ||
| 3 | 1 (7.7%) | 7 (4.7%) | 29 (8.0%) | 35 (10.4%) | 21 (13.4%) | 17 (12.7%) | 110 (9.5%) | |
| 4 | 3 (2.0%) | 11 (3.0%) | 19 (5.7%) | 25 (15.9%) | 12 (9.0%) | 70 (6.1%) | ||
| 5 | 4 (2.7%) | 4 (1.1%) | 10 (3.0%) | 8 (5.1%) | 16 (11.9%) | 42 (3.6%) | ||
| 6 | 1 (0.7%) | 12 (3.3%) | 35 (10.4%) | 20 (12.7%) | 37 (27.6%) | 105 (9.1%) | ||
| No data | 1 (0.7) | 6 (1.7%) | 6 (1.8%) | 2 (1.3%) | 15 (1.3%) | |||
| Total | 13 (100%) | 149 (100%) | 363 (100%) | 336 (100%) | 157 (100%) | 134 (100%) | 1152 (100%) | |
Patients with facial paresis before surgery and the patients that died within one year postoperatively are not in the table. Percentages are percentages within each column.
Figure 1.
Bar chart showing the percentages of patients with a good (House-Brackmann grade 1 and 2) and poor outcome (House-Brackmann grade 3 to 6) facial function one year after surgery in relation to tumor growth and size (patients with missing data are excluded).
In Table 3, the facial function 1 year after the surgery is related to the facial function at discharge. Out of 1117 patients with data on facial function available at both time points 747 (66.8%) had some degree of facial paresis at discharge. Yet, in 579 (77.5%) of these patients the paresis improved over time, in 316 (42.3%) cases from a poor facial function to a good function. Conversely, in 24 patients (2.1%) the facial function deteriorated over time, and in 13 of these patients from a good facial outcome to a poor outcome. The risk of a progressing facial paresis was not related to tumor size group (N = 1117, χ2 = 1.477, p = 0.831).
Table 3. Facial Nerve Function after 1 Year based on Facial Function at Hospital Discharge.
| House-Brackmann Grade At Discharge from Hospital | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | No Data | |||
| House-Brackmann grade 1 year after surgery | 1 | 361 (97.6%) | 95 (79.2%) | 101 (63.9%) | 28 (32.9%) | 20 (19.8%) | 20 (7.0%) | 1 (5.6%) | 626 (55.0%) |
| 2 | 3 (0.8%) | 18 (15.0%) | 37 (23.4%) | 31 (36.5%) | 33 (32.7%) | 46 (16.0%) | 4 (22.2%) | 172 (15.1%) | |
| 3 | 3 (0.8%) | 6 (5.0%) | 16 (10.1%) | 14 (16.5%) | 19 (18.8%) | 49 (17.1%) | 2 (11.1%) | 119 (9.6%) | |
| 4 | 3 (1.9%) | 8 (9.4%) | 16 (15.8%) | 43 (15.0%) | 70 (6.1%) | ||||
| 5 | 1 (0.3%) | 1 (0.8%) | 1 (1.2%) | 12 (11.9%) | 27 (9.4%) | 42 (3.7%) | |||
| 6 | 2 (0.5%) | 1 (0.6%) | 2 (2.4%) | 1 (1.0%) | 99 (34.5%) | 105 (9.2%) | |||
| No data | 1 (1.2%) | 3 (1.0%) | 11 (61.1%) | 15 (1.3%) | |||||
| Total | 370 (100%) | 120 (100%) | 158 (100%) | 85 (100%) | 101 (100%) | 287 (100%) | 18 (100%) | 1139 (100%) | |
Patients with intrameatal tumors or preoperative facial paresis and patients who died within 1 year postoperatively are not included in the table. Percentages are percentages within each column.
Discussion
Patients
This is the largest series of patients who have undergone translabyrinthine surgery for VS presented to date. The series covers a long time period of 33 years and therefore also changes in surgical equipment and surgical techniques. Furthermore, seven different surgeons have performed the operations making it probable that some of the variation in results is explained by other factors than those described in details above. However, the high number of patients and the relatively high follow-up rates increase the scientific value of the study.
The mean age of the patients in this study was 53 years and corresponds to that presented in other larger series.6,16,17,18 The mean size of the tumors with extrameatal extention was 25 mm, and parallels that described in other larger studies not focusing solely on larger tumors.6,17,19,20 We present the pure extrameatal size of the tumors not including the part of the tumor in the internal auditory canal.14 Other groups have used the combined intra- and extrameatal diameter to describe tumor size, which makes direct comparison between results complex and with a tendency to overestimate the facial results in studies where tumor in the internal auditory canal is included in the size estimation. Whether this has influenced the somewhat inferior facial nerve outcome presented in this study compared with some other larger series6,17,20 is unknown. The preoperative hearing level of the patients in this study corresponds to that presented by Brackmann.6 Approximately 14% of the patients with preoperative audiograms available had Class A hearing according to the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS), and ~9% had speech discrimination loss (DL) = 0%. The latter group of patients would today, if the size of the tumor was small, only be recommended surgery if growth had been established, and in that case hearing preserving surgery would be suggested.13,21 In other patients with preoperative serviceable hearing, recommendations concerning the surgical approach are less obvious as data suggest that a large percentage of patients with any DL >0% will lose serviceable hearing within a few years irrespectively of the treatment.13,21
Tumor Removal
In 84% of the patients the tumor was completely removed, and in ~86% of the patients with incomplete tumor removal only small remnants were left unresected. Direct comparison with other studies is difficult since few studies follow the recommendations for reporting tumor removal.14 Still, these results are consistent with other larger studies reporting 85 to 98% complete tumor removal.6,16,17,19,22 Tumor adherence to the facial nerve was the primary reason that complete tumor removal was not attained, and the likelihood of incomplete resection increased with increasing tumor size. Interestingly, the long-term facial function of the patients with incomplete resection was not different from that of the patients with complete resection. As these patients inherently have more tumor involvement with the nerve and still demonstrate similar long-term facial function it supports the surgical decision to leave small tumor remnants to preserve facial nerve function. Others have reported equivalent data to support this opinion.6 We currently follow the growth patterns of these small tumor remnants with serial MRI and will publish the results as soon as sufficient long-term data are available. The general impression is that the growth potential of such remnants is very limited as indicated by other smaller studies.23,24,25,26 This is an essential observation, as revision surgery has been shown to bear a higher risk of facial damage than primary surgery.23 Whether secondary stereotactic radiotherapy will be a better alternative in these patients is still unresolved.27
Facial Nerve Preservation
In ~85% of the patients the facial nerve was assessed as intact during surgery, and in ~8% the nerve was assessed as intact but thin. The probability of an anatomical intact facial nerve not surprisingly decreased with increasing tumor size. Again direct comparison with other studies is difficult as no consensus on reporting results exists, but still the rates seem to match those reported by others.6,16,17 The 78 patients with disrupted facial nerves were over the years handled with different strategies, but it is today also our general opinion that the patients are best managed by direct end-to-end anastomosis, or if that is not possible by primary cable graft.28 We presently further investigate the long-term facial results of these patients, and the results will be published separately.
Complications
The patients who died from complications of surgery were generally older than the overall population, which supports an attitude toward “wait and scan” in this group of patients if possible. Also, the size of the tumors of the patients who died was generally larger than in the overall population, with a third of the patients having giant tumors, confirming that surgery of larger posterior fossa tumors may be precarious. Still, data from other recent studies6,16,20 demonstrate that 0% mortality can be achieved even in larger tumors – at least in smaller selected materials. However, it must be remembered that this study is unique since it presents all translabyrinthine surgeries for VS in a country. To minimize the risk of late complications from postoperative hematomas we have used routine early postoperative control computed tomography scans for some years now. In case of a significant hematoma in the surgical cavity reoperation is recommended to avoid later neurological deterioration. Furthermore, today all surgeries are performed at the Copenhagen University Hospital with neurosurgical and neurointensive care unit services and the postoperative neurointensive observation level has been intensified for all patients.
The ~14% risk of postoperative CSF leaks in this study is somewhat higher than reported by others,6,10,17 but lower than that found in cohorts of patients with tumors larger than 30 mm.16,25,29 Over the years, we have combined the use of a free fat grafts with blind closure of the middle ear and eustachian tube, meticulous closure of the temporalis fascia, and an autologous fibrin sealant (Vivostat, Vivostat, Denmark), and it is our opinion that this has decreased the risk of CSF leaks and especially almost removed the problem with wound leaks. We do not routinely use lumbar drainage. Our data indicate, that if a CSF leak occur, most wound leaks can be managed conservatively or with lumbar drains, whereas patients with rhinoliquorrhea often require revision surgery.
Long-Term Facial Function
Data from this study support the general finding that facial outcome after translabyrinthine surgery is highly depending on the size of the tumor.6,16,18,19 Overall, ~70% had a good outcome, and these results are comparable to those reported by some authors,17,18,19,30 but somewhat inferior to those reported by others.6,17,31 Again, the problem with different ways of reporting tumor size should be taken into consideration, and it is recommended that future series follow the international recommendations as we have done here.
The general clinical experience is that in patients with larger tumors the facial nerve is thin and fragile, and trying to attain complete tumor removal may very well be on the expense of an intact facial nerve and thereby the chance of a good facial outcome. This further supports the surgical strategy of leaving microscopic tumor remnants on the facial nerve if it is judged that the nerve will be damaged by further dissection. Some authors suggest staged procedures for large tumors32 with primary debulking via a retrosigmoid approach and secondary translabyrinthine resection of residual tumor, but in the majority of these cases small tumor remnants were anyhow left on the facial nerve. An alternative approach is primary surgical debulking followed by stereotactic radiotherapy,33,34 but too little data are available to evaluate the outcome of such schemes. Besides, it is our general experience that tumors of any size can be removed via the translabyrinthine approach, and we share concern about radiotherapy in the treatment of histological benign tumors in younger patients.6 Nevertheless, larger tumor remnants often grow,23,24,35 and from a practical clinical perspective some form of plan for follow-up or secondary procedures should be available for such patients.
This study confirms that the majority of the patients with an early postoperative facial paresis will spontaneously improve over time.6,16 This supports longer clinical follow-up before secondary facial nerve repair is considered in patients with an anatomically intact facial nerve. Also, it should be taken into consideration that the best facial function attainable after secondary facial nerve repair is HB grade 3,6,36 which should be compared with the existing facial function of the patient and the symptom relieving effects of cosmetic surgery and/or eye lid surgery to attain eye closure.37
In this study, only few patients experienced a progressing facial paresis, and though some authors suggest using steroids in these cases the effect of such treatment is not based on evidence from larger clinical studies.38,39 Also, some authors suspect viral reactivation of a latent infection, similar to the proposed mechanism of Bell's palsy, as the cause of progressive paresis and suggest antiviral prophylactic.6,38,39,40 Again, such treatment is currently not based on hard evidence.
Conclusion
In this large series of patients, the translabyrinthine approach proved efficient in tumor control and with satisfactory facial nerve outcome. Good long-term facial function was obtained in the majority of the patients with smaller tumors, and the majority of the facial paresis observed in the early postoperative period spontaneously improved during the following months. This observation is important, as it implies the need for a long observation period before facial reanimation procedures are considered in patients with anatomically intact nerves. With larger tumors the risk of a persistent facial paresis is more evident, and more data on patients managed by alternative strategies are warranted. Leaving small tumor remnants on the facial nerve in “difficult tumors” to preserve integrity of the nerve seems reasonable, as these patients in general will have the same chance of a good facial outcome as patients with complete tumor removal. Furthermore, preliminary results suggest that the growth potential of such small remnants is limited. Over 33 year, we have experienced 12 cases where the patient died from direct complication to surgery. These patients were generally older and had larger tumors than the average patient. This calls for a conservative attitude toward surgery in elderly patients, and further demonstrates the need for more data on alternative management strategies in larger tumors. Also, it demonstrates the need for intensive clinical observation in the early postoperative period, and the possibility to react rapidly if signs of serious complications should occur. After translabyrinthine surgery the risk of postoperative CSF leakage is limited, and most wound leaks can be managed conservatively or with lumbar drains. Conversely, about half the patients with rhinoliquorrhea will need a secondary procedure. Fortunately, modifications in our surgical closure technique seem to have decreased that risk of CSF leakage. Based on these observations, we still consider the use of the translabyrinthine approach for tumors of any size in cases where functional hearing preservation is not an issue.
References
- 1.Thomsen J, Tos M, Møller H, Charabi S. The choice of approach in surgery for acoustic neuromas (vestibular schwannomas) Tokai J Exp Clin Med. 1994;19(3-6):93–101. [PubMed] [Google Scholar]
- 2.Stangerup S-E, Tos M, Thomsen J, Caye-Thomasen P. Hearing outcomes of vestibular schwannoma patients managed with ‘wait and scan’: predictive value of hearing level at diagnosis. J Laryngol Otol. 2010;124(5):490–494. doi: 10.1017/S0022215109992611. [DOI] [PubMed] [Google Scholar]
- 3.Pollock B E Driscoll C LW Foote R L et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery Neurosurgery 200659177–85., discussion 77–85 [DOI] [PubMed] [Google Scholar]
- 4.Myrseth E, Møller P, Pedersen P-H, Lund-Johansen M. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64(4):654–661, discussion 661–663. doi: 10.1227/01.NEU.0000340684.60443.55. [DOI] [PubMed] [Google Scholar]
- 5.Tos M, Thomsen J. The translabyrinthine approach for the removal of large acoustic neuromas. Arch Otorhinolaryngol. 1989;246(5):292–296. doi: 10.1007/BF00463578. [DOI] [PubMed] [Google Scholar]
- 6.Brackmann D E, Cullen R D, Fisher L M. Facial nerve function after translabyrinthine vestibular schwannoma surgery. Otolaryngol Head Neck Surg. 2007;136(5):773–777. doi: 10.1016/j.otohns.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol. 1998;255(1):1–6. doi: 10.1007/s004050050012. [DOI] [PubMed] [Google Scholar]
- 8.Stangerup S-E, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. doi: 10.1258/0022215041917989. [DOI] [PubMed] [Google Scholar]
- 9.Slattery W H, Francis S, House K C. Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol. 2001;22(6):895–902. doi: 10.1097/00129492-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Selesnick S H, Liu J C, Jen A, Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25(3):387–393. doi: 10.1097/00129492-200405000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Brackmann D E, Green J D. Translabyrinthine approach for acoustic tumor removal. 1992. Neurosurg Clin N Am. 2008;19(2):251–264, vi. doi: 10.1016/j.nec.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Merkus P, Taibah A, Sequino G, Sanna M. Less than 1% cerebrospinal fluid leakage in 1,803 translabyrinthine vestibular schwannoma surgery cases. Otol Neurotol. 2010;31(2):276–283. doi: 10.1097/MAO.0b013e3181cc06ad. [DOI] [PubMed] [Google Scholar]
- 13.Stangerup S-E, Caye-Thomasen P, Tos M, Thomsen J. Change in hearing during ‘wait and scan’ management of patients with vestibular schwannoma. J Laryngol Otol. 2008;122(7):673–681. doi: 10.1017/S0022215107001077. [DOI] [PubMed] [Google Scholar]
- 14.Kanzaki J, Tos M, Sanna M, Moffat D A, Monsell E M, Berliner K I. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642–648, discussion 648–649. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 15.House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 16.Lanman T H, Brackmann D E, Hitselberger W E, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999;90(4):617–623. doi: 10.3171/jns.1999.90.4.0617. [DOI] [PubMed] [Google Scholar]
- 17.Fayad J N, Schwartz M S, Slattery W H, Brackmann D E. Prevention and treatment of cerebrospinal fluid leak after translabyrinthine acoustic tumor removal. Otol Neurotol. 2007;28(3):387–390. doi: 10.1097/01.mao.0000265188.22345.d4. [DOI] [PubMed] [Google Scholar]
- 18.Bouetel V, Lescanne E, François P, Jan M, Morinière S, Robier A. [Evolution of facial nerve prognosis in vestibular schwannoma surgery by translabyrinthine approach] Rev Laryngol Otol Rhinol (Bord) 2008;129(1):27–33. [PubMed] [Google Scholar]
- 19.Mass S C, Wiet R J, Dinces E. Complications of the translabyrinthine approach for the removal of acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1999;125(7):801–804. doi: 10.1001/archotol.125.7.801. [DOI] [PubMed] [Google Scholar]
- 20.Shamji M F, Schramm D R, Benoit B G. Clinical predictors of facial nerve outcome after translabyrinthine resection of acoustic neuromas. Clin Invest Med. 2007;30(6):E233–E239. doi: 10.25011/cim.v30i6.2951. [DOI] [PubMed] [Google Scholar]
- 21.Stangerup S-E, Thomsen J, Tos M, Cayé-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol. 2010;31(2):271–275. doi: 10.1097/MAO.0b013e3181c34bda. [DOI] [PubMed] [Google Scholar]
- 22.Roche P-H, Pellet W, Moriyama T, Thomassin J-M. Translabyrinthine approach for vestibular schwannomas: operative technique. Prog Neurol Surg. 2008;21:73–78. doi: 10.1159/000156708. [DOI] [PubMed] [Google Scholar]
- 23.El-Kashlan H K, Zeitoun H, Arts H A, Hoff J T, Telian S A. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. 2000;21(3):389–392. doi: 10.1016/s0196-0709(00)80049-6. [DOI] [PubMed] [Google Scholar]
- 24.Bloch D C, Oghalai J S, Jackler R K, Osofsky M, Pitts L H. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130(1):104–112. doi: 10.1016/S0194-5998(03)01598-5. [DOI] [PubMed] [Google Scholar]
- 25.Godefroy W P, der Mey A GL van, de Bruine F T, Hoekstra E R, Malessy M JA. Surgery for large vestibular schwannoma: residual tumor and outcome. Otol Neurotol. 2009;30(5):629–634. doi: 10.1097/MAO.0b013e3181a8651f. [DOI] [PubMed] [Google Scholar]
- 26.Talfer S, Dutertre G, Conessa C, Desgeorges M, Poncet J-L. Surgical treatment of large vestibular schwannomas (stages III and IV) Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(2):63–69. doi: 10.1016/j.anorl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Roche P-H, Ribeiro T, Khalil M, Soumare O, Thomassin J M, Pellet W. Recurrence of vestibular schwannomas after surgery. Prog Neurol Surg. 2008;21:89–92. doi: 10.1159/000156711. [DOI] [PubMed] [Google Scholar]
- 28.Arriaga M A, Brackmann D E. Facial nerve repair techniques in cerebellopontine angle tumor surgery. Am J Otol. 1992;13(4):356–359. [PubMed] [Google Scholar]
- 29.Mamikoglu B, Wiet R J, Esquivel C R. Translabyrinthine approach for the management of large and giant vestibular schwannomas. Otol Neurotol. 2002;23(2):224–227. doi: 10.1097/00129492-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Devèze A, Roche P-H, Facon F, Gabert K, Pellet W, Thomassin J M. [Functional outcomes after translabyrinthine approach for vestibular schwannomas] Neurochirurgie. 2004;50(2-3 Pt 2):244–252. [PubMed] [Google Scholar]
- 31.Sughrue M E, Yang I, Rutkowski M J, Aranda D, Parsa A T. Preservation of facial nerve function after resection of vestibular schwannoma. Br J Neurosurg. 2010;24(6):666–671. doi: 10.3109/02688697.2010.520761. [DOI] [PubMed] [Google Scholar]
- 32.Patni A H, Kartush J M. Staged resection of large acoustic neuromas. Otolaryngol Head Neck Surg. 2005;132(1):11–19. doi: 10.1016/j.otohns.2004.09.094. [DOI] [PubMed] [Google Scholar]
- 33.Fuentes S, Arkha Y, Pech-Gourg G, Grisoli F, Dufour H, Régis J. Management of large vestibular schwannomas by combined surgical resection and gamma knife radiosurgery. Prog Neurol Surg. 2008;21:79–82. doi: 10.1159/000156709. [DOI] [PubMed] [Google Scholar]
- 34.Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59(4):283–289, discussion 289–291. doi: 10.1016/s0090-3019(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 35.Sakaki S, Nakagawa K, Hatakeyama T, Murakami Y, Ohue S, Matsuoka K. Recurrence after incompletely resected acousticus neurinomas. Med J Osaka Univ. 1991;40(1-4):59–66. [PubMed] [Google Scholar]
- 36.Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve—preservation and restitution of function. Neurosurgery. 1997;40(4):684–694, discussion 694–695. doi: 10.1097/00006123-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Tos T, Caye-Thomasen P, Stangerup S-E, Thomsen J, Tos M. Need for facial reanimation after operations for vestibular schwannoma: patients perspective. Scand J Plast Reconstr Surg Hand Surg. 2003;37(2):75–80. doi: 10.1080/02844310310005595. [DOI] [PubMed] [Google Scholar]
- 38.Darrouzet V, Martel J, Enée V, Bébéar J-P, Guérin J. Vestibular schwannoma surgery outcomes: our multidisciplinary experience in 400 cases over 17 years. Laryngoscope. 2004;114(4):681–688. doi: 10.1097/00005537-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen D Q, Franco-Vidal V, Guérin J, Darrouzet V. [Delayed facial palsy after vestibular schwannoma resection: the role of viral reactivation. Our experience in 8 cases] Rev Laryngol Otol Rhinol (Bord) 2004;125(1):23–29. [PubMed] [Google Scholar]
- 40.Magliulo G, D'Amico R, Di Cello P. Delayed facial palsy after vestibular schwannoma resection: clinical data and prognosis. J Otolaryngol. 2003;32(6):400–404. doi: 10.2310/7070.2003.13968. [DOI] [PubMed] [Google Scholar]