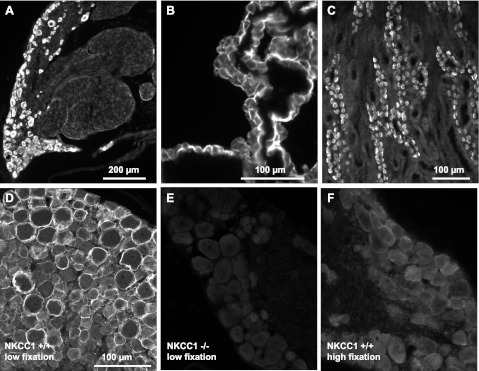
Fig. 2.
Validation of NKCC1 antibody specificity. A–C: the polyclonal antibody (Kaplan-CT), raised in rabbit against amino acids 938–1011 of the carboxy terminus of mNKCC1, selectively labels all neurons in rat DRG (A) and also selectively labels rat tissues known to express NKCC1 such as the apical membrane of rat choroid plexus epithelial cells (B) and the basolateral membrane of α-intercalated cells of the outer medullary collecting duct of rat kidney (C). D: NKCC1 immunofluorescence of DRG neurons from wild-type mouse (NKCC1+/+) obtained with the Kaplan-CT antibody. Note that the antibody preferentially labels the surface of neurons and that the most intense immunofluorescence frequently surrounds the cells of larger diameter. This NKCC1 immunoreactivity (IR) is typically obtained when the tissues are prepared in “low fixation” conditions (i.e., no postfixation following perfusion-fixation with 4% paraformaldehyde). NKCC1-IR is totally absent in DRGs from NKCC1−/− mice (E) and is very significantly decreased in NKCC1+/+ DRGs postfixed in 4% paraformaldehyde for ≥2 h after perfusion-fixation (F, “high fixation”). This suggests that NKCC1 epitope is sensitive to fixation protocols (compare D, low postfixation, with F, high fixation). Scale bar in D applies to E and F.