Abstract
The central auditory system consists of the lemniscal and nonlemniscal pathways or systems, which are anatomically and physiologically different from each other. In the thalamus, the ventral division of the medial geniculate body (MGBv) belongs to the lemniscal system, whereas its medial (MGBm) and dorsal (MGBd) divisions belong to the nonlemniscal system. Lemniscal neurons are sharply frequency-tuned and provide highly frequency-specific information to the primary auditory cortex (AI), whereas nonlemniscal neurons are generally broadly frequency-tuned and project widely to cortical auditory areas including AI. These two systems are presumably different not only in auditory signal processing, but also in eliciting cortical plastic changes. Electric stimulation of narrowly frequency-tuned MGBv neurons evokes the shift of the frequency-tuning curves of AI neurons toward the tuning curves of the stimulated MGBv neurons (tone-specific plasticity). In contrast, electric stimulation of broadly frequency-tuned MGBm neurons augments the auditory responses of AI neurons and broadens their frequency-tuning curves (nonspecific plasticity). In our current studies, we found that electric stimulation of AI evoked tone-specific plastic changes of the MGBv neurons, whereas it degraded the frequency tuning of MGBm neurons by inhibiting their auditory responses. AI apparently modulates the lemniscal and nonlemniscal thalamic neurons in quite different ways. High MGBm activity presumably makes AI neurons less favorable for fine auditory signal processing, whereas high MGBv activity makes AI neurons more suitable for fine processing of specific auditory signals and reduces MGBm activity.
Keywords: corticofugal modulation, frequency tuning, medial geniculate body, nonspecific plasticity, tone-specific plasticity
the central auditory system of mammals consists of the lemniscal and nonlemniscal systems. In the thalamus, the lemniscal system is composed of the ventral division of the medial geniculate body (MGBv) that projects to the primary auditory cortex (AI) and anterior auditory field (AAF). The nonlemniscal system is composed of the medial (MGBm) and dorsal (MGBd) divisions of the MGB and the posterior intralaminar nucleus. The MGBd projects to the cortical auditory areas surrounding AI, whereas the MGBm projects to all auditory areas including AI (Imig and Morel 1983 for review; Andersen et al. 1980; Winer et al. 1977). The cortical auditory areas project back to the subcortical auditory nuclei: AI and AAF project back to the MGBv; AII to MGBd; and all auditory areas, including AI, to MGBm (Andersen et al. 1980; Rouiller 1997 for review; Winer et al. 2001). The MGBm must be more important than the MGBd for the interaction between the lemniscal and nonlemniscal systems. The thalamic reticular nucleus (TRN) receives collateral projections from the thalamocortical (Crabtree 1998; Jones 1975) and corticothalamic fibers and projects to the surrounding thalamic nuclei (He 2003; Hu 2003; Pinault 2004; Rouiller 1997 for reviews). These anatomic connections and neurophysiological studies indicate that focal electric stimulation of AI modulates not only the MGBv, but also MGBm for auditory signal processing and reorganization through the TRN (Zhang et al. 2008).
In general, the MGBv is tonotopically organized by sharply frequency-tuned neurons, whereas the MGBm and MGBd are poorly tonotopically organized by broadly frequency-tuned neurons. The MGBm is polysensory. The responses of MGBm and MGBd neurons are highly variable and quickly habituate to identical stimuli (Aitkin and Webster 1972; Clarey et al. 1992 for reviews). However, they are sensitive to changes in auditory stimuli (Kraus et al. 1994).
Electric stimulation of the guinea pig AI (He et al. 2002; He 2003; Yu et al. 2004; Zhang et al. 2008) and ACh applied to the rat auditory thalamus (Mooney et al. 2004) excite MGBv neurons but inhibit MGBm neurons. It is most likely that both AI and ACh differently modulate MGBv and MGBm neurons for auditory signal processing. Ma and Suga (2009) found that AI neurons show tone-specific plasticity [best-frequency (BF) shifts] for electric stimulation of the MGBv but nonspecific plasticity (nonspecific augmentation) for that of the MGBm. In our current studies, we found that focal electric stimulation of AI evokes the BF shifts of sharply tuned MGBv neurons and the degradation of the auditory responses of broadly tuned MGBm neurons.
MATERIALS AND METHODS
Materials, surgery, acoustic stimulation, electric stimulation, recording of action potentials, and data acquisition and processing were the same as those described in Ma and Suga (2001, 2009), although the sites of electric simulation and recording auditory responses (action potential) were respectively AI and the MGB instead of the MGB and AI. Therefore, only the essential portions of the methods are described below. The Animal Studies Committee of Washington University in St. Louis approved all experimental procedures.
Sixteen adult big brown bats (Eptesicus fuscus) were used for the current experiments. Under neuroleptanalgesia (4.08 mg/kg body wt; Innovar), a 1.5-cm-long metal post was glued on the dorsal surface of the bat's skull. Physiological experiments were started 3–4 days after the surgery. The awake animal was placed in a polyethylene-foam body-mold that was hung with an elastic band at the center of a soundproof room maintained at 31°C. The metal post glued on the skull was fixed to a metal rod with set screws to immobilize the animal's head, and the head was adjusted to face directly toward a loudspeaker located 74 cm away. Several holes (∼100 μm in diameter) were made in the skull covering AI. A reference tungsten-wire electrode with a ∼15-μm tip diameter was placed on the cortical surface at one of these holes. A recording tungsten-wire electrode with a ∼7-μm tip diameter was inserted into AI through the other holes for recording neural responses to tone bursts at 300- to 700-μm depths, and the approximate center of AI (tuned to 25–30 kHz) and the posterior edge of AI were located. Then, a pair of tungsten-wire electrodes that were separated by ∼110 μm horizontally and 50–150 μm vertically at their tips was inserted at the center for electric stimulation. After recording the auditory responses and measuring the BF and frequency-tuning curves of AI neurons, this pair of electrodes was connected to the constant current stimulus isolator for electric stimulation. A four-electrode assembly (Ma and Suga 2009; NeuroNexus Technologies, Ann Arbor, MI) was inserted at the posterior edge of AI into the MGBm (1.3–1.5 mm lateral to the midline) or MGBv (1.8–2.1 mm lateral to the midline) through the hippocampus for recording the auditory responses of MGB neurons so that we first observed the responses of cortical auditory neurons, regular spontaneous discharges of hippocampus neurons, and then the auditory responses of MGBm neurons (1.1–1.3 mm below the hippocampus) or MGBv neurons (0.8–1.0 mm below it). An electrode of the four-electrode assembly that did not record auditory responses was used as a reference electrode. The effects of the cortical electric stimulation on the auditory response and frequency tuning of broadly tuned MGBm or sharply tuned MGBv neuron were then studied.
Acoustic stimulation.
For the measurement of the frequency-threshold or -response curve and the BF of a MGBm, MGBv, or AI neuron, a 4.0-ms tone burst, including a 0.5-ms rise-decay time, was delivered to the animal from a leaf tweeter (Model EAS-10 TH-800; Panasonic) at a rate of 4/s. Its frequency and amplitude were varied manually or computer-controlled (TDT System 3; Tucker-Davis Technologies). Before the experiments, the sound amplitude was calibrated with a Brüel and Kjæel ¼-in. calibrated microphone (Model #4135; Copenhagen, Denmark) placed at the location of the bat's ears and was expressed in decibels in sound-pressure level (dB SPL) referencing to 20-Pa root-mean-square.
The frequency-threshold (tuning) curve of a MGB or an AI neuron was first audiovisually measured. Then, the tone burst was randomly changed in amplitude in 5-dB steps from 0 to 100 dB SPL and in frequency in 0.5-kHz steps between ±5.0 kHz of the BF if the tuning curve was narrow but between ±10 kHz of the BF if it was broad by TDT System 3. The frequency-amplitude scan was repeated 10 times to obtain 10 responses at each frequency-amplitude combination. It took 7 or 14 min to complete the 10 scans. A frequency-threshold curve of a neuron was obtained from its responses to the frequency-amplitude scans. The frequency-response curve of the neuron could be obtained by counting the number of action potentials at 30 dB above the minimum threshold (MT) of the neuron as a function of frequency. However, the frequency-response curves based on 50 responses at a given frequency were obtained with the frequency scan at 30 dB above the MT delivered 50 times.
Action potentials of AI and MGB neurons.
Action potentials recorded usually originated from a few neurons. Therefore, we used an amplitude-window-discriminator software (Tucker-Davis Technologies) to select action potentials of a single neuron. At the beginning of data acquisition, the waveform of an action potential was stored and displayed on the monitor screen. The data acquisition was continued as long as other action potentials obtained during data acquisition matched with this action potential (i.e., template). Since the electrode placements and recording action potentials were exactly the same as those in Ma and Suga's experiments (2009), the recording sites in the MGB were not anatomically studied in our current experiments.
Electric stimulation.
An electric stimulator (S88; Grass Technologies) and a constant current isolator (A360; WPI) were used for focal electric stimulation of AI. A 6.2-ms train of four monophasic electric pulses (100-nA constant current, 0.2-ms duration, 2.0-ms interval) was delivered to AI neurons at a rate of 10/s for 30 min through the paired tungsten-wire electrodes as described above. The bat showed no behavioral response at all to such weak electric stimulation.
Data acquisition and processing.
Data obtained from the MGB before and after the focal electric stimulation of AI were stored on a computer hard drive and were used for offline data processing that included plotting frequency-threshold and -response curves and measuring the BF, MT, and quality factor, Q-30 dB, which was the BF divided by the tuning width at 30 dB above the MT of a given neuron. The frequency-threshold curves of the thalamic neurons could be classified into either sharply tuned or multipeaked broadly tuned according to both their shapes and Q-30 dB.We calculated a Q-30 dB instead of a Q-10 dB because the Q-10 dB was related to the tuning width only at the tip portion of a tuning curve and did not appropriately represent the difference in sharpness between broadly tuned and sharply tuned frequency-tuning curves. The broadly tuned neuron had a Q-30 dB <6.5, and the sharply tuned neuron had a Q-30 dB >6.5, as described in results. The paired t-test was used to test the difference between the responses obtained before and after the electric stimulation and also between the broadly tuned and sharply tuned thalamic neurons.
RESULTS
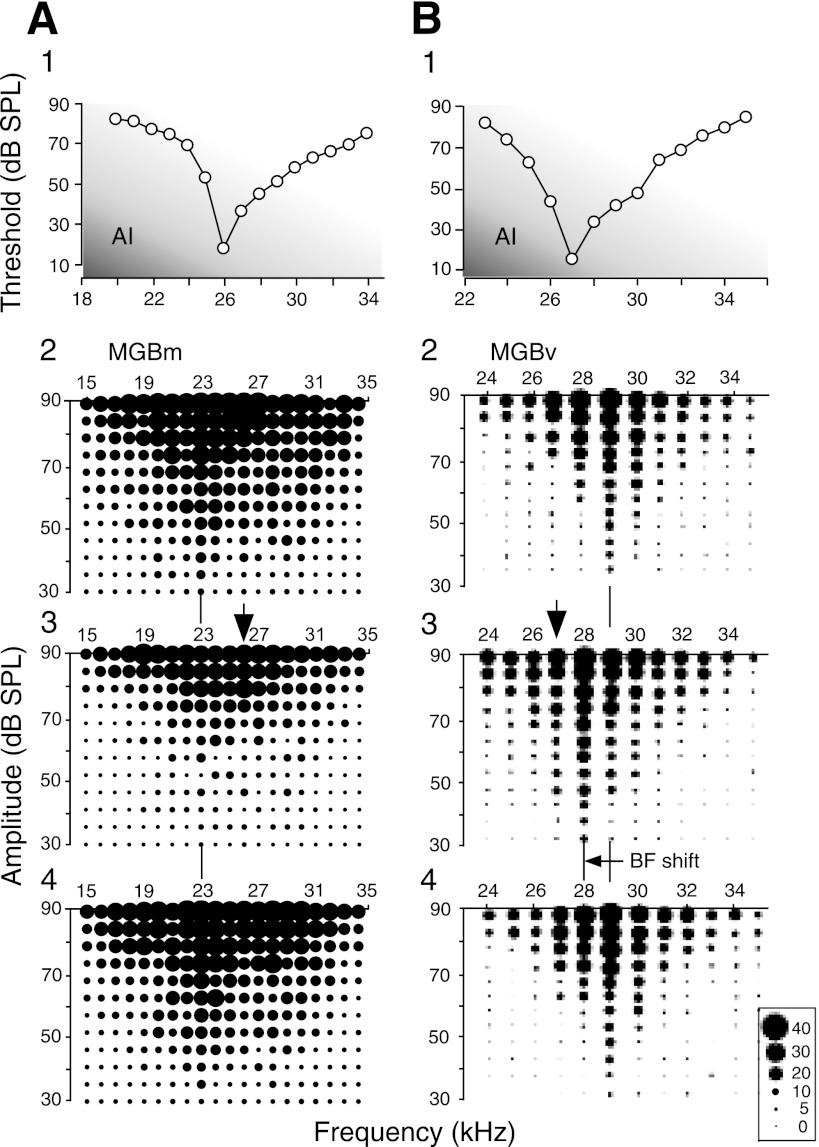
The BFs of cortical (AI) neurons recorded ranged from 24.0 to 54.0 kHz (mean ± SD: 36.0 ± 9.3 kHz, n = 51). On the other hand, those of MGB neurons recorded ranged from 20.0 to 55.0 kHz (38.0 ± 9.5 kHz, n = 51). Corticofugal modulation was different depending on the sharpness of the frequency-threshold curves of thalamic auditory neurons. Figure 1 displays the frequency-threshold curves of two cortical neurons (A1 and B1) and the response areas (receptive fields) of a MGBm (A2–4) and a MGBv (B2–4) neuron. The cortical neurons were tuned to either 26.0 or 27.0 kHz. Their Q-30 dB were either 7.4 or 9.0. The MGBm neuron in Fig. 1A2 was broadly tuned and multipeaked. Its BF and Q-30 dB were respectively 23.0 kHz and 1.3. On the other hand, the MGBv neuron in Fig. 1B2 was sharply tuned to 29.0 kHz, and its Q-30 dB was 11.6. The broadly tuned MGBm neuron was medially located, whereas the sharply tuned MGBv one was located laterally.
Fig. 1.
Changes in the response areas of broadly tuned MGBm and sharply tuned MGBv neurons (MGBm and MGBv: the medial and ventral divisions of the medial geniculate body, respectively). A1 and B1: the frequency-threshold (tuning) curves of 2 cortical [primary auditory cortex (AI)] neurons electrically stimulated. A2 and B2: the response areas of a MGBm and a MGBv neuron in the control condition, respectively. A3 and B3: the response areas of these thalamic neurons changed by electric stimulation of the cortical neurons shown in A1 and B1, respectively. The change was either a decrease in response (A3) or best-frequency (BF) shift (B3). A4 and B4: recovery of the response areas recorded ∼60 min after the onset of the cortical stimulation. The vertical bars and arrows indicate the BFs of the thalamic and cortical neurons, respectively. The scale at the bottom right indicates the number of action potentials discharged by the single neuron to 10 identical tonal stimuli. dB SPL, decibels in sound-pressure level.
When the cortical neuron (Fig. 1A1) was electrically stimulated, the responses of the broadly tuned MGBm neuron (Fig. 1A2) to tone bursts were suppressed, its response area was reduced, its threshold became higher, and its Q-30 dB reduced from 1.3 to 1.1 (Fig. 1A3). That is, the response properties of the neuron were “degraded.” The effects of the cortical stimulation disappeared ∼60 min after the onset of the electric stimulation (A4). Such suppression of the responses of a thalamic neuron occurred over many frequencies even if the BF of the electrically stimulated cortical neurons was different as much as 24 kHz from that of the thalamic neuron. Therefore, the suppression was not specific to the BF of the stimulated cortical neurons. Such nonspecific suppression or inhibition was observed in all 34 broadly tuned MGBm neurons studied.
When the cortical neuron (Fig. 1B1) was electrically stimulated, the response area of the MGBv neuron sharply tuned to 29.0 kHz (Fig. 1B2) shifted its response area toward the BF of the stimulated neuron so that the BF of the neuron changed from 29.0 to 28.0 kHz. The width of the response area (tuning curve) of this thalamic neuron slightly widened (Fig. 1B3) so its Q-30 dB changed from 11.6 to 9.3. The shifted BF returned to the control BF ∼60 min after the onset of the electric stimulation (Fig. 1B4). The BF shift, i.e., tuning shift was generally based on a decrease in response by inhibition at the BF of the recorded neuron in the control condition and an increase in response (facilitation) at the frequencies at or near the BF of the stimulated cortical neuron (Ma and Suga 2004; Xiao and Suga 2002, 2004, 2005). Such a BF shift evoked by AI stimulation was observed in 15 sharply tuned neurons out of the 17 studied. The BF shift is also elicited by auditory fear conditioning and has been known as a tone-specific plastic change (Suga and Ma 2003 for review). In the following, the nonspecific suppression and BF shift evoked by the cortical stimulation are further documented with the frequency-threshold and -response curves obtained from thalamic neurons.
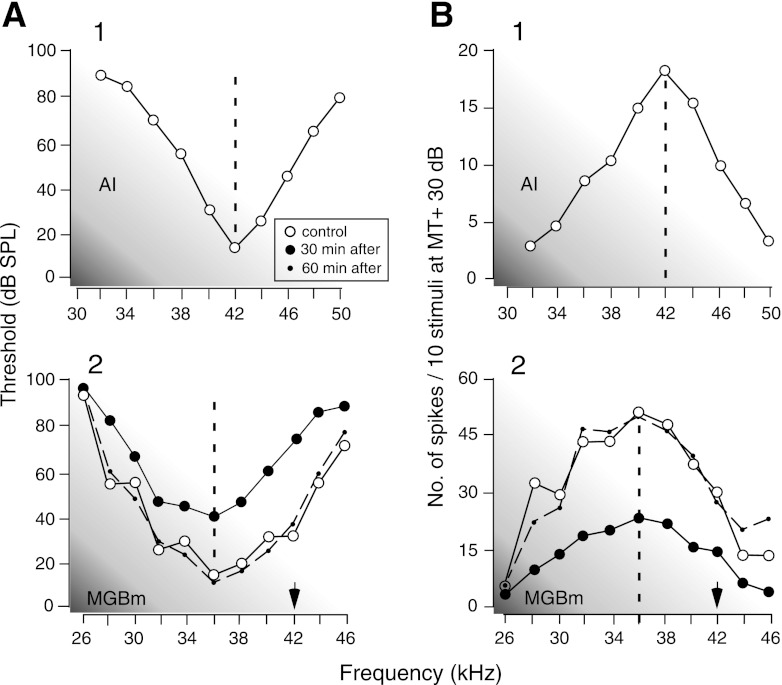
In Fig. 2, A1 and B1 show the frequency-threshold and -response curves of a cortical neuron, respectively. Its BF was 42.0 kHz. Electric stimulation of this neuron evoked nonspecific suppression of a MGBm neuron broadly tuned to 36.0 kHz with a Q-30 dB of 2.9 (Fig. 2, A2 and B2, open circles). That is, the frequency-threshold curve of the thalamic neuron became 30 dB higher (Fig. 2A2, closed circles), and the responses to tone bursts became 57% less at the BF (Fig. 2B2, closed circles). The suppression of the response occurred at many different frequencies so that it was nonspecific to frequencies. The amount of the decrease in the response over the 10-kHz band with the BF at its center was 51%. The BF did not shift. These changes in the curves and responses reverted to the control condition ∼60 min after the onset of the cortical electric stimulation (Fig. 2, A2 and B2, dashed lines).
Fig. 2.
Changes in the frequency-threshold (A) and frequency-response (B) curves of a broadly tuned MGBm neuron evoked by electric stimulation of cortical neurons. The cortical stimulation (A1 and B1) evoked an increase in the frequency-threshold curve (A2) and a decrease in frequency-response (B2) curve of the MGBm neuron. The open and closed circles represent the curves in the control condition and 30 min after the onset of the cortical stimulation, respectively. The dashed curves represent the data obtained 60 min after the onset of the electric stimulation. The frequency-response curves were measured at 30 dB above the minimum threshold of the MGBm neuron. The arrows indicate the BFs of the stimulated neurons.
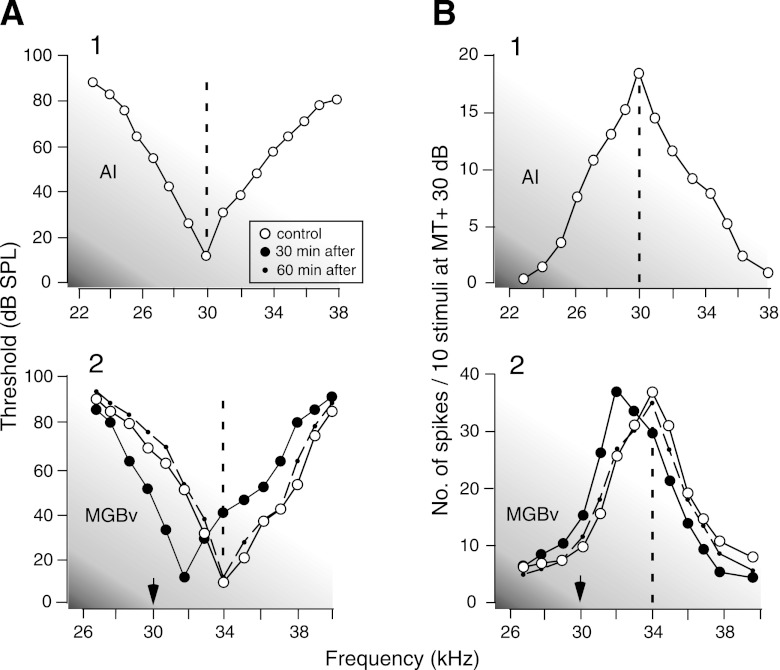
The corticofugal effect on the sharply tuned thalamic neurons was quite different from the above. Figure 3 shows the frequency-threshold (AI) and -response (B1) curves of a cortical neuron tuned to 30.0 kHz. When this neuron was electrically stimulated, the BF of the sharply tuned MGBv neuron (Fig. 3, A2 and B2, open circles) shifted from 34.0 to 32.0 kHz (Fig. 3, A2 and B2, closed circles). That is, the BF shifted toward the BF of the stimulated cortical neuron. The shifted BF reverted to the control BF ∼60 min after the onset of the electric stimulation (A2 and B2, dashed lines).
Fig. 3.
Changes in the frequency-threshold (A) and frequency-response (B) curves of a sharply tuned MGBv neuron evoked by cortical stimulation. The cortical stimulation (A1 and B1) laterally shifted the frequency-threshold (A2) and frequency-response (B2) curves of the MGBv neuron. MT, minimum threshold.
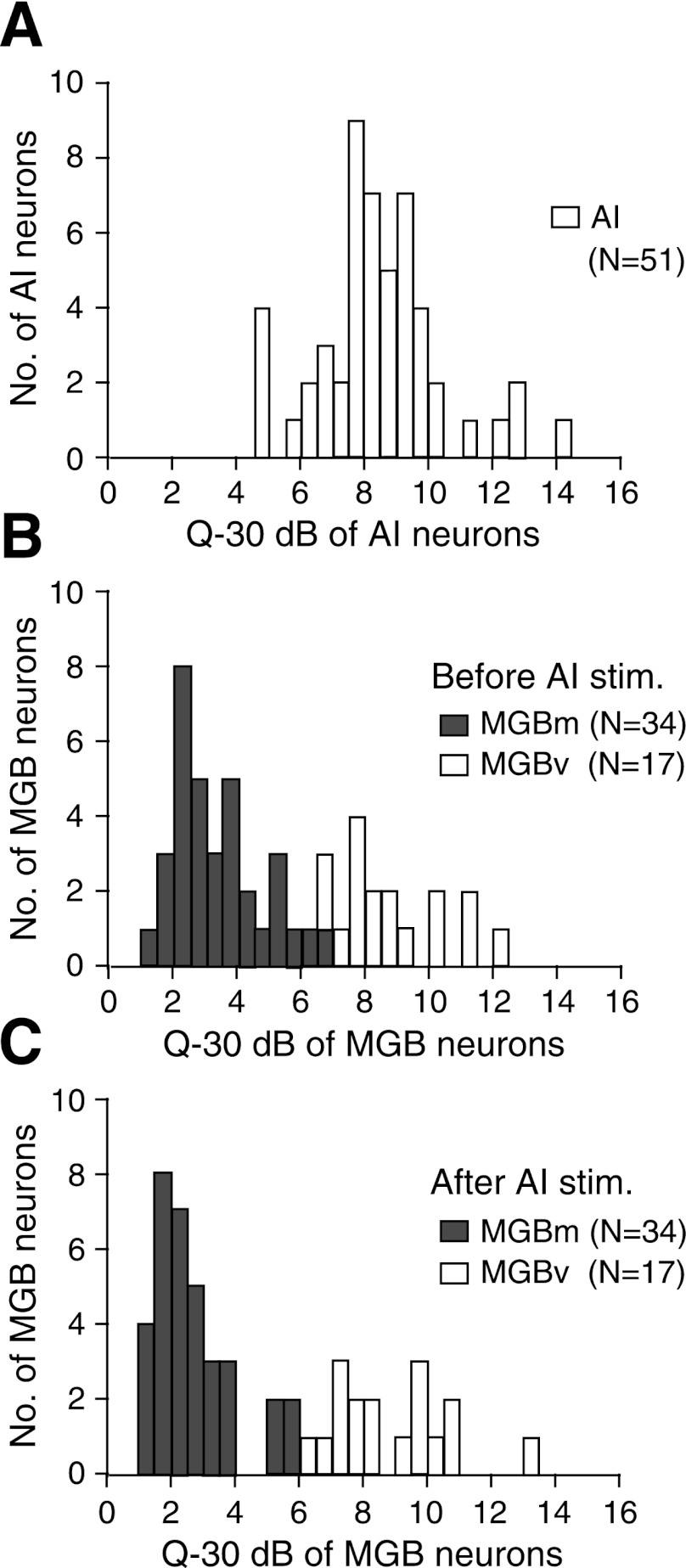
The thalamic neurons were classified as either broadly or sharply tuned according to the shapes of their tuning curves and their Q-30 dB. The distributions of the Q-30 dB of the 51 stimulated cortical and 51 recorded thalamic neurons are shown in Fig. 4, A and B, respectively. The Q-30 dB of the 51 cortical neurons, except 4, were unimodally distributed from 5.8 to 14.5 (8.3 ± 2.0). On the other hand, those of the 51 thalamic neurons were bimodally distributed from 1.3 to 12.0. The 1st and 2nd groups, respectively, corresponded to the MGBm and MGBv neurons. Since the distributions crossed over at the Q-30 dB of 6.5, the tuning curves with the Q-30 dB <6.5 were defined as broadly tuned, and those with the Q-30 dB larger than that were defined as sharply tuned. There were 3 neurons with Q-30 dB between 6.5 and 7.9. One of them was the MGBm neuron, and the remaining two were the MGBv neurons. As described later, this MGBm neuron reduced its response by 27%, increased its MT by 20 dB, and decreased its Q-30 dB by 8% after the cortical electric stimulation, as the typical broadly tuned MGBm neurons. On the other hand, the two MGBv neurons showed a response change <8%, a MT change <5 dB, and a Q-30 dB change <4% after the cortical electric stimulation, as the typical sharply tuned neurons.
Fig. 4.
Distributions of the Q-30 dB of stimulated cortical (A) and recorded thalamic neurons (B and C). A: the Q-30 dB of the stimulated cortical neurons. B and C: the Q-30 dB of thalamic neurons before and after the cortical stimulation (stim.), respectively. The white and gray bars, respectively, indicate the Q-30 dB of MGBm and MGBv neurons. Q-30 dB is the BF divided by the bandwidth of a tuning curve at 30 dB above the minimum threshold of a given neuron.
The Q-30 dB of the 34 MGBm neurons ranged between 1.3 and 6.5 (3.3 ± 1.4) with a mode at 2.0–2.5, and their BFs ranged from 20.0 to 54.0 kHz (37.0 ± 9.5 kHz). The Q-30 dB of the MGBv neurons ranged from 6.5 to 12.0 (8.7 ± 1.7) with a mode at 7.5–8.0 (Fig. 4B), and their BFs ranged from 26.0 to 55.0 kHz (38.0 ± 9.8 kHz). The BFs were not different between these 34 MGBm and 17 MGBv neurons (P> 0.5). Therefore, the difference in Q-30 dB between these two groups was not due to any difference in BF.
Recording MGBv neurons was much easier than that of MGBm neurons. However, we studied them every three to four neurons because the corticofugal modulation of the sharply tuned MGBv neurons had already been studied in the mustached bat (Zhang and Suga 2000) and the house mouse (Zhang and Yan 2008) and because the corticofugal modulation of sharply tuned collicular neurons of the big brown bat had already been known to be BF shifts as in the above two species of animals (Ma and Suga 2001). When broadly tuned and sharply tuned neurons were simultaneously recorded with an electrode assembly, they were always at the electrodes located medially and laterally, respectively.
After cortical stimulation, the Q-30 dB of the 34 broadly tuned neurons ranged between 1.1 and 5.8 (2.6 ± 1.2). They were significantly smaller than those in the control condition (P< 0.001; Fig. 4C, gray bars). Their BFs ranged between 20.0 and 54.0 kHz (38 ± 9.6 kHz) and were not different from those in the control condition (P> 0.5). After the cortical stimulation, however, the Q-30 dB of the 17 sharply tuned neurons ranged between 6.6 and 13.0 (9.0 ± 1.9). They were not different from those in the control condition (Fig. 4C, white bars). The BFs of 15 neurons out of the 17 shifted after the cortical stimulation. The remaining 2 did not show a BF shift: 1 was BF-matched with the stimulated cortical neurons, and the other had a BF that was 10 kHz higher than that of the stimulated neurons.
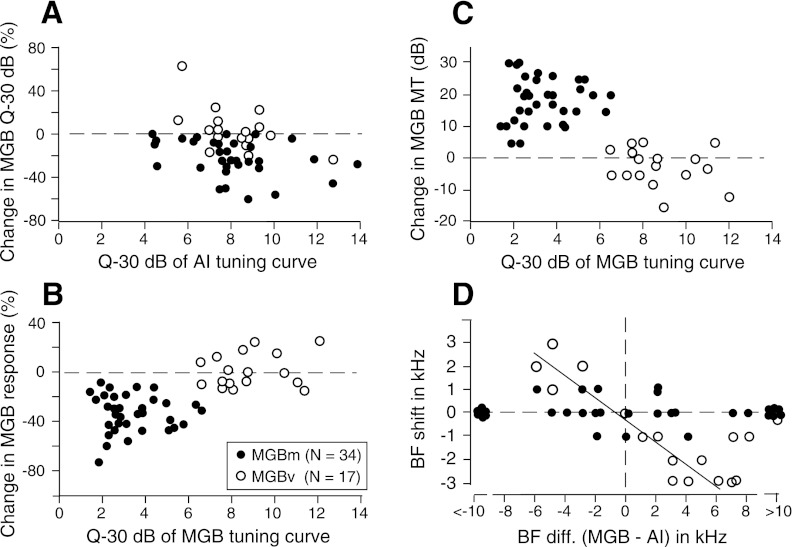
Changes in the Q-30 dB of the individual thalamic neurons elicited by cortical electric stimulation are plotted in Fig. 5A as a function of the Q-30 dB of the stimulated cortical neurons. The cortical stimulation broadened the frequency-threshold curves of the broadly tuned MGBm neurons. The decrease in a Q-30 dB ranged between 1 and 58% (22 ± 16%, n = 34). In general, the cortical AI neurons “degraded” the response properties of the broadly tuned MGBm neurons, as further explained below. On the other hand, the cortical stimulation showed no change or slight increase or decrease in the Q-30 dB of the sharply tuned MGBv neurons. The mean change in Q-30 dB was 4.0 ± 2.0% (n = 17). The corticofugal effects on the MGBm and MGBv neurons were quite different (P< 0.001). This difference was not related to the Q-30 dB of the stimulated cortical neurons.
Fig. 5.
Changes in the sharpness (Q-30 dB) of the frequency-threshold curves (A), the response magnitudes (B), and the minimum thresholds (C) of thalamic neurons as a function of the sharpness of stimulated cortical (AI) or recorded thalamic (MGB) neurons and the BF shifts of the thalamic neurons as a function of the BF differences (diff.) between the recorded thalamic and stimulated cortical neurons (D). Changes in the response magnitude of the thalamic neurons were calculated with the total numbers of spikes discharged in a 10-kHz band with the BF of a given neuron at its center. Closed and open circles: the data obtained from the MGBm and MGBv neurons, respectively. In D, the regression line is for the open circles within ±6.0-kHz BF differences (r2=0.89).
The most prominent and easily detectable change evoked by the cortical stimulation was that in the magnitude of a response (number of spikes) to a tone-burst stimulation. The percent change in the responses in a 10-kHz band with the BF of a given neuron at its center was counted before and after the cortical stimulation, and the percent change in response was calculated. The changes ranged between −8 and −76% (−34 ± 16%, n = 34) for the broadly tuned MGBm neurons and between −15 and +27% (+2.1 ± 14%, n = 17) for the sharply tuned MGBv neurons (Fig. 5B). The difference in the response change between the two groups of thalamic neurons was not related to the BFs (P> 0.5) but to the sharpness of the tuning curves. It was clear that the broadly tuned MGBm neurons were corticofugally inhibited, whereas the sharply tuned MGBv neurons were not. This was the second difference in corticofugal modulation between the MGBm and MGBv neurons. The third difference was the change in MT. That is, the change in a MT was between 0 and 30 dB (18.5 ± 6.9 dB, n = 34) for the broadly tuned MGBm neurons and between −15 and +5 dB (−2.3 ± 5.8 dB, n = 17) for the sharply tuned MGBv ones (Fig. 5C). This difference was statistically significant (P< 0.01). The cortical stimulation increased the thresholds of the MGBm neurons but tended to decrease the thresholds of the MGBv neurons.
The fourth difference in corticofugal modulation between the MGBm and MGBv neurons was a tuning shift, i.e., a BF shift. In Fig. 5D, the BF shifts of thalamic neurons are plotted as a function of the BF differences between the recorded thalamic and stimulated cortical neurons. It has been known that BF shifts depend on the difference in BF between the recorded and stimulated auditory neurons (Suga and Ma 2003 for review). In the big brown bat, they were rarely observed when the difference is >10 kHz and when the electric stimulation pulse is weak, 0.2 ms, 100 nA (Ma and Suga 2001). Therefore, the BF shift that might be elicited by cortical stimulation was particularly examined for the thalamic neurons with a BF within a range of ±10 kHz of the BFs of the stimulated cortical neurons. In the MGBm, the BF increased 1.0 kHz in 5 neurons and decreased 1.0 kHz in 3 neurons. The BF did not change in the remaining 26 neurons (Fig. 5D, closed circles). We could not find any systematic BF shift of the MGBm neurons as the function of the BF difference between the stimulated and recorded neurons. On the contrary, the BFs of the MGBv neurons systematically shifted as a function of the difference in the BF between the stimulated and recorded neurons. The correlation coefficient (r) was 0.9 for ± 6.0 kHz BF differences. The amount of the BF shift became smaller at BF differences >6.0, as reported by Ma and Suga (2001) and Yan and Suga (1998).
DISCUSSION
Sharpness of the frequency-tuning curves of MGBm neurons.
In general, MGBm neurons are broadly frequency-tuned, whereas MGBv neurons are sharply frequency-tuned in cats (Aitkin 1973; Calford 1983), guinea pigs (Zhang et al. 2008), and big brown bats (Ma and Suga 2009). In the rat thalamic auditory neurons, Bordi and LeDoux (1994) found that the distribution of Q-10 dB overlapped between the MGBm and MGBv neurons and wrote that “Overall, frequency tuning functions were narrower in MGBv than in the other [thalamic auditory] areas, but many cells in MGBm were as narrowly tuned as cells in MGBv”. One might say that some MGBv cells were as broadly tuned as some MGBm cells. A Q-10 dB is inappropriate to express the overall sharpness of the tuning curves of central auditory neurons because their tuning curves are commonly not simple triangular shape and because Q-10 dB is based on the bandwidth of a tuning curve only at its tip portion. Calford (1983) used a scale based on the bandwidth of the tuning curve at 20 dB above the MT. Since auditory neurons commonly respond to tone bursts over 60–80 dB, we calculated a Q-30 dB measuring the bandwidth at 30 dB above the MT. Terms such as sharp, narrow, wide, and broad are arbitrary, so someone's narrow can be another's broad.
In our current data, the Q-30 dB of the MGBm neurons ranged between 1.3 and 6.5, and those of the MGBv neurons ranged between 6.5 and 12.0. We called the tuning curves with a Q-30 dB <6.5 broadly tuned and those with a Q-30 dB larger than that sharply tuned. The neurons with a Q-30 dB at or near the boundary can be called either broadly or narrowly tuned by changing the definition.
Corticofugal modulation of MGBm and MGBv neurons.
In the guinea pig, most on-responding MGBv neurons are excited by AI stimulation, whereas the great majority of on-responding MGBm neurons are inhibited by it (He 2003; Yu et al. 2004). The corticofugal inhibition of MGBm neurons is evoked through the TRN (Zhang et al. 2008). Unlike the above excitatory or inhibitory corticofugal effect evoked by intense electric stimulation of AI, focal weak electric stimulation of AI evokes both frequency-dependent facilitation and inhibition and BF shifts of neurons in AI (Chowdhury and Suga 2000; Ma and Suga 2001), the MGBv (Zhang et al. 1997; Zhang and Suga 2000; current study), the inferior colliculus central nucleus (ICc; Jen and Zhou 2003; Ma and Suga 2001; Yan and Ehret 2002; Yan and Suga 1998; Zhang et al. 1997; Zhang and Suga 2000), and cochlear nucleus (Luo et al. 2008). When a recorded subcortical or cortical neuron is matched in BF to stimulated cortical neurons, the response of the matched neuron is augmented at its BF and is inhibited at frequencies lower and/or higher than the BF. As a result, its frequency tuning is sharpened. When BF-unmatched, the unmatched neuron is inhibited at its BF and is facilitated at the frequencies at and near the BF of the stimulated neurons. As a result, its frequency tuning shifts, i.e., its BF shifts. All these corticofugal modulations have been studied only in the lemniscal system (Suga and Ma 2003 and Suga 2012 for review). Our current data obtained from the big brown bat indicate that AI stimulation degrades the response properties of broadly tuned MGBm neurons by suppressing their responses to tone bursts, increasing their thresholds and broadening their frequency-tuning curves and that it evokes the BF shift of sharply tuned MGBv neurons. Cortical electric stimulation would activate multiple neural pathways that might evoke the thalamic changes. The most likely pathway for these changes is, however, AI to the MGBv for excitation and AI to the MGBm through the TRN for inhibition, as reported by He et al. (2002) and Zhang et al. (2008). Since AI stimulation evokes the BF shifts of subthalamic auditory neurons (Suga 2012 for review), the BF shifts of MGBv neurons must be partly due to those subthalamic BF shifts.
Corticofugal modulation of auditory thalamus and plasticity elicited by conditioning or pseudoconditioning.
Our current findings are presumably related to the plasticity elicited by auditory fear conditioning and the differential gating mechanism, as discussed below. Auditory fear conditioning with paired conditioning tone (CS) and unconditioned leg (US) stimuli elicits the large long-lasting AI and large short-lasting ICc, MGBv, and MGBm BF shifts (Suga and Ma 2003 and Weinberger 2004 for review). Tone-burst stimulation associated with electric stimulation of the basal nucleus evokes the BF shifts of MGBv neurons through AI (Zhang and Yan 2008). Focal electric stimulation of AI evokes BF shifts in not only AI and MGBv, but also subthalamic auditory nuclei (Suga 2012 for review). These BF shifts occur in a specific relation to the BF of the stimulated neurons or the frequency of the conditioning tone. Therefore, they are a tone-specific plastic change. When the CS and US are unpaired for pseudoconditioning, however, AI, MGBv, and ICc neurons show nonspecific augmentation (sensitization), which is quite different from BF shifts (Bakin et al. 1992; Ji and Suga 2008, 2009).
Focal electric stimulation of the MGBv evokes the BF shifts of AI neurons similar to those elicited by conditioning, whereas that of the MGBm evokes the nonspecific augmentation of auditory responses and broadening of the frequency-tuning curves of AI neurons similar to those elicited by pseudoconditioning (Ma and Suga 2009). Our current data indicate that focal electric stimulation of AI degrades MGBm neurons, whereas it evokes the BF shifts of MGBv neurons. These findings indicate that AI neurons are improved for fine auditory signal processing by the thalamocortical and corticothalamic feedback loop through the lemniscal MGBv neurons and that, at the same time, they suppress the activity of nonlemniscal MGBm neurons and make them inappropriate for fine auditory signal processing. Electric stimulation of AI excites most MGBv neurons and inhibits the great majority of MGBm neurons (Yu et al. 2004) through the TRN (Zhang et al. 2008). ACh depolarizes MGBv neurons but hyperpolarizes MGBm neurons (Mooney et al. 2004). It is possible that a high level of MGBm activity makes AI neurons somewhat similar to nonlemniscal neurons and unfavorable for fine auditory signal processing. Considering all these neural events, Suga (2008) suggested the presence of a differential gating mechanism and also the implication of the corticofugal system, TRN, and neuromodulators to this mechanism.
The US activates the somatosensory system, the ascending reticular activating system (ARAS), and the brain aversion system (BAS). The BAS and ARAS both activate the histaminergic system that broadly releases histamine (HA) in the brain and play an important role in eliciting defensive behaviors and arousal, respectively (Siegel 2002 for review). HA may play an important role in eliciting the nonspecific augmentation and in suppressing the BF shifts (W. Ji and N. Suga, unpublished observations). It has been known that ACh play an essential role in eliciting BF shifts due to cortical electric stimulation (Ma and Suga 2005) and auditory fear conditioning (Weinberger 1998 for review; Ji et al. 2001; Ji and Suga 2003). Thus ACh and HA both are presumably involved in the differential gating mechanism, if it exists.
GRANTS
This work was supported by a research grant from the National Institute on Deafness and Other Communication Disorders (DC-000175).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.T. and W.Y. performed experiments; J.T., W.Y., and N.S. analyzed data; J.T., W.Y., and N.S. interpreted results of experiments; J.T., W.Y., and N.S. prepared figures; J.T., W.Y., and N.S. drafted manuscript; J.T., W.Y., and N.S. approved final version of manuscript; N.S. conception and design of research; N.S. edited and revised manuscript.
REFERENCES
- Aitkin LM. Medial geniculate body of the cat: responses to tonal stimuli of neurons in medial division. J Neurophysiol 36: 275–283, 1973 [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Webster WR. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol 35: 365–380, 1972 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Knight PL, Merzenich MM. The thalamocortical and corticothalamic connections of AI, AII, and the anterior auditory field (AAF) in the cat: evidence for two largely segregated systems of connections. J Comp Neurol 194: 663–701, 1980 [DOI] [PubMed] [Google Scholar]
- Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res 577: 226–235, 1992 [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res 98: 261–274, 1994 [DOI] [PubMed] [Google Scholar]
- Calford MB. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci 3: 2350–2364, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, Suga N. Reorganization of the tonotopic map of the auditory cortex evoked by cortical electrical stimulation in the big brown bat. J Neurophysiol 83: 1856–1863, 2000 [DOI] [PubMed] [Google Scholar]
- Clarey JC, Barone P, Imig TJ. Physiology of thalamus and cortex. In: The Mammalian Auditory Pathway: Neurophysiology, edited by Popper AN, Fay RR. New York: Springer-Verlag, 1992, chapt. 5, p. 232–334 [Google Scholar]
- Crabtree JW. Organization in the auditory sector of the cat's thalamic reticular nucleus. J Comp Neurol 390: 167–182, 1998 [PubMed] [Google Scholar]
- He J. Corticofugal modulation of the auditory thalamus. Exp Brain Res 153: 579–590, 2003 [DOI] [PubMed] [Google Scholar]
- He J, Yu YQ, Xiong Y, Hashikawa T, Chan YS. Modulatory effect of cortical activation on the lemniscal auditory thalamus of the guinea pig. J Neurophysiol 88: 1040–1050, 2002 [DOI] [PubMed] [Google Scholar]
- Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res 153: 543–549, 2003 [DOI] [PubMed] [Google Scholar]
- Imig TJ, Morel A. Organization of the thalamocortical auditory system in the cat. Annu Rev Neurosci 6: 95–120, 1983 [DOI] [PubMed] [Google Scholar]
- Jen PH, Zhou X. Corticofugal modulation of amplitude domain processing in the midbrain of the big brown bat, Eptesicus fuscus. Hear Res 184: 91–106, 2003 [DOI] [PubMed] [Google Scholar]
- Ji W, Gao E, Suga N. Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol 86: 211–225, 2001 [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol 90: 1904–1909, 2003 [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Tone-specific and nonspecific plasticity of auditory cortex elicited by pseudoconditioning: role of acetylcholine receptors and the somatosensory cortex. J Neurophysiol 100: 1384–1396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Tone-specific and nonspecific plasticity of inferior colliculus elicited by pseudoconditioning: role of acetylcholine and auditory and somatosensory cortices. J Neurophysiol 102: 941–952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol 162: 285–308, 1975 [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change. J Neurophysiol 72: 1270–1277, 1994 [DOI] [PubMed] [Google Scholar]
- Luo F, Wang Q, Kashani A, Yan J. Corticofugal modulation of initial sound processing in the brain. J Neurosci 28: 11615–11621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Lateral inhibition for center-surround reorganization of the frequency map of bat auditory cortex. J Neurophysiol 92: 3192–3199, 2004 [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proc Natl Acad Sci USA 102: 9335–9340, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Plasticity of bat's central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J Neurophysiol 85: 1078–1087, 2001 [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Specific and nonspecific plasticity of the primary auditory cortex elicited by thalamic auditory neurons. J Neurosci 29: 4888–4896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney DM, Zhang L, Basile C, Senatorov VV, Ngsee J, Omar A, Hu B. Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci USA 101: 320–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev 46: 1–31, 2004 [DOI] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: The Central Auditory System, edited by Ehret G, Ramand R.New York: Oxford Univ. Press, 1997, p. 3–96 [Google Scholar]
- Siegel J. The Neural Control of Sleep and Waking. New York: Springer-Verlag, 2002 [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Comp Physiol A 194: 169–183, 2008 [DOI] [PubMed] [Google Scholar]
- Suga N. Tuning shifts of the auditory system by corticocortical and corticofugal projections and conditioning. Neurosci Biobehav Rev 36: 969–988, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783–794, 2003 [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem 70: 226–251, 1998 [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5: 279–290, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Diamond IT, Raczkowski D. Subdivisions of the auditory cortex of the cat: the retrograde transport of horseradish peroxidase to the medial geniculate body and posterior thalamic nuclei.J Comp Neurol 176: 387–417, 1977 [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ, Larue DT. Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol 430: 27–55, 2001 [PubMed] [Google Scholar]
- Xiao Z, Suga N. Asymmetry in corticofugal modulation of frequency-tuning in mustached bat auditory system. Proc Natl Acad Sci USA 102: 19162–19167, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the auditory cortex specialized forecho-delay processing in the mustached bat. Proc Natl Acad Sci USA 101: 1769–1774, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat's auditory system by inhibition. Proc Natl Acad Sci USA 99:15743–15748, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Ehret G. Corticofugal modulation of midbrain sound processing in the house mouse. Eur J Neurosci 16: 119–128, 2002 [DOI] [PubMed] [Google Scholar]
- Yan W, Suga N. Corticofugal modulation of the midbrain tonotopic map in the bat auditory system. Nat Neurosci 1: 54–58, 1998 [DOI] [PubMed] [Google Scholar]
- Yu YQ, Xiong Y, Chan YS, He J. Corticofugal gating of auditory information in the thalamus: an in vivo intracellular recording study. J Neurosci 24: 3060–3069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suga N. Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bat. J Neurophysiol 84: 325–333, 2000 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature 387: 900–903, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu CH, Yu YQ, Fujimoto K, Chan YS, He J. Corticofugal projection inhibits the auditory thalamus through the thalamic reticular nucleus. J Neurophysiol 99: 2938–2945, 2008 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan J. Corticothalamic feedback for sound-specific plasticity of auditory thalamic neurons elicited by tones paired with basal forebrain stimulation. Cereb Cortex 18: 1521–1528, 2008 [DOI] [PubMed] [Google Scholar]