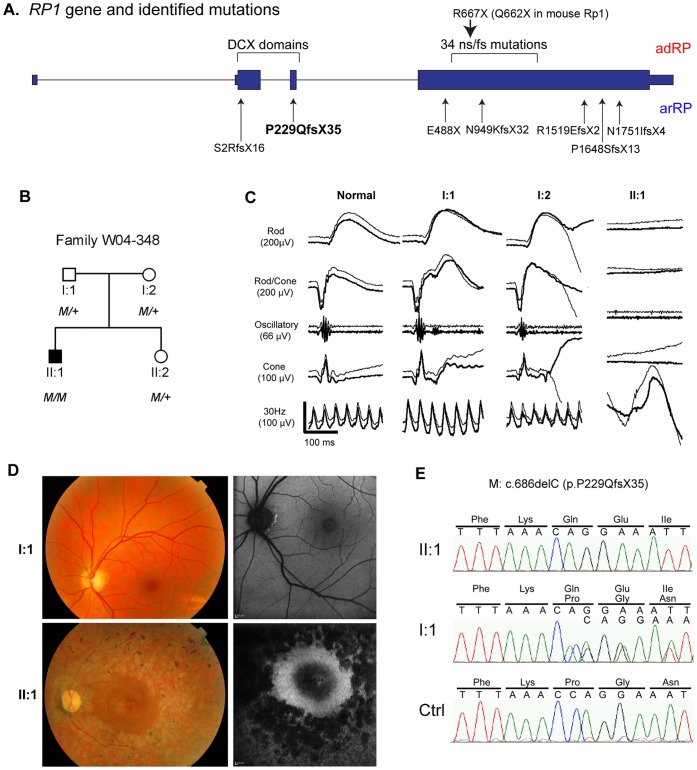
Figure 1. RP1 Gene, Clinical and Sequence Data for Family W04-348.
A. RP1 gene and identified mutations. The gene structure of RP1 is depicted, with the locations of mutations that cause adRP and arRP indicated; the mutations above the RP1 gene structure cause dominant RP, labeled as adRP in red; whereas mutations below the RP1 gene structure cause recessive RP, labeled as arRP in blue; frameshift mutation p.P229QfsX35 reported to cause arRP in this study is in bold. The portion of the gene that encodes that DCX domains is also indicated. The arrow on the top indicates the location of the R677X (human) and Q662X (mouse) mutations. B. Pedigree for family W04-348. The c.686delC, p.P229QfsX35 mutation is designated by M. C. ERG traces from a normal control, the patient’s parents (I-1, I-2 (age 57), and the affected patient (II-1). The five standard ISCEV recordings are shown, from the top including: scotopic rod responses, scotopic combined rod-cone responses, oscillatory potentials, photopic single flash and photopic 30Hz responses. The amplitude and time scales are indicated. The ERG responses of the patient’s parents show normal amplitudes and implicit times; the patient had no recordable rod or cone responses. The deflections shown in the 30 Hz recordings for the patient are due to motion artifact. The thicker traces are from the right eye, the thinner from the left eye. D. Fundus photos (left) and fundus autofluorescence images (right) of the affected patient II-1(age 30) and his father I-1 (age 60). The patient has typical findings of RP, with optic disc pallor, attenuation of the retinal blood vessels, RPE atrophy and bone spicule pigmentation outside the macula. As shown in the autofluorescence image, the RPE in the macular region is relatively preserved. In contrast, the father’s fundi are normal. E. Sequence traces showing the homozygous c.686delC mutation in patient II-1, carrier status of this mutation in the patient’s father I-1, and the wild-type sequence in an unaffected Dutch control. Note that the sequence trace of the mutant allele in individual I-1 is shifted slightly.