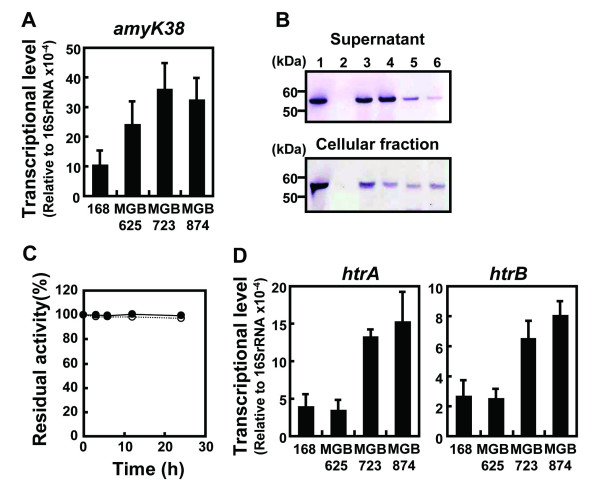
Figure 2.
Investigation of the cause of decreased AmyK38 production.B subtilis strains 168 (a), MGB625 (b), MGB723 (c), and MGB874 (d) harboring pHYK38 were cultured in 2xL-Mal medium with shaking at 30°C. (A) Transcriptional levels of amyK38 were determined by qRT-PCR after 48 h of cultivation and are reported relative to those of 16 S rRNA. (B) Secretion and cellular amounts of AmyK38 in the genome-reduced strains. The concentrations of AmyK38 in supernatants and cellular fractions after 48 h of cultivation were analyzed by Western blotting. Supernatants were diluted 40-fold and 3 μl was used for analysis. Cells were re-suspended to an OD600 of 3.0, corresponding to an approximately 10-fold dilution of cultures, 12 μl samples were used for analysis. Thus, cellular fractions were 16-fold enriched compared to supernatants. The positions of the protein standards are indicated in kDa to the left of the gel. Lanes: 1, purified AmyK38 (10 ng); 2, strain 168 harboring pHY300PLK (empty vector); 3, strain 168 harboring pHYK38; 4, strain MGB625 harboring pHYK38; 5, strain MGB723 harboring pHYK38; and 6, strain MGB874 harboring pHYK38. (C) Stability of AmyK38 in spent culture medium. The spent culture media of strains 168 (open circles) and MGB874 harboring pHYK38 (closed circles) were collected after 48 h of cultivation. Time intervals of α-amylase activities of the spent media were measured at 30°C. (D) Transcriptional levels of htrA and htrB after 48 h of cultivation were determined by qRT-PCR and are reported relative to those of 16 S rRNA. The results presented in (A) and (D) are the means of three individual experiments. Error bars represent standard deviations (n = 3).