Abstract
Systemic injection of lipopolysaccharide (LPS) induces a robust immune response as well as thermal and mechanical hyperalgesia. Spinal and peripheral glial cells have been implicated as important mediators in this hyperalgesia but the specific contributions of microglia versus astrocytes are not entirely clear. To better define these mechanisms, this study examined the febrile response, nociceptive sensitivity, glial cell reactivity and cytokine production in the dorsal root ganglion (DRG) and spinal cord in rats following systemic treatment with LPS and the effects of minocycline in countering these responses. Intraperitoneal LPS injection resulted in an increase in core body temperature and produced hyperalgesia to heat and mechanical stimuli. Western blot studies revealed increased expression of microgial cell, macrophage and satellite cell markers in DRG and microglial and astrocyte markers in spinal cord following LPS treatment. Real-time RT-PCR indicated that LPS treatment increased cytokine mRNA expression levels in both the DRG and the spinal cord. Minocycline suppressed all LPS-induced behavioral effects but not the febrile response. Moreover, minocycline prevented LPS induced microglia/macrophage activation and cytokine responses in spinal cord and DRG, but did not affect the activation of astrocytes/satellite cells. These data demonstrate that LPS-induced changes in nociceptive sensitivity are likely mediated by activation of microglial cells and/or macrophages in the spinal cord and DRG.
Keywords: Lipopolysaccharide, Minocycline, Hyperalgesia, Dorsal root ganglion, Spinal cord, Inflammation, Microglia, Astrocyte, Satellite cell
Introduction
Lipopolysaccharide (LPS), a major component of the outer membrane of gram-negative bacteria induces most clinical aspects of acute bacterial infection, including inflammation and changes in immune functions when introduced systemically that lead to systemic illness response such as anorexia, fever and lethargy (Dantzer et al.,1998). These effects of LPS are all dependent on the induction of pro-inflammatory cytokines. Moreover, intraperitoneal injection of LPS or specific cytokines enhances nociceptive sensitivity to various somatic stimuli in rodents suggesting hyperalgesia (Maier et al.,1993). Because nociceptive sensitivity is mediated by neural circuitry in the spinal cord or brain, this hyperalgesia appears to be part of the CNS response to peripherally injected LPS.
Ascending nociceptive information passes through the peripheral dorsal root ganglion (DRG) to the spinal cord and then hence to the brain (Woolf,2004). Various inflammatory mediators such as cytokines, eicosanoids, and nitric oxide increase the sensitivity of the DRG and spinal cord to nociceptive signals, thereby reducing thresholds for nociceptive stimuli in acute and chronic pain models (Woolf,2004). Glial cells, as the main source of cytokines in the CNS, are active participants in the neuroimmune response to a variety of stressors (Wieseler-Frank et al.,2004). However, the relative contribution of glial subtypes, microglia versus astrocytes, appears to vary in hyperalgesia with differing etiologies. Microglial cells appear to play a key role in hyperalgesia produced by direct nerve injuries (Milligan et al.,2003; Raghavendra et al.,2003), whereas astrocytes appear to play the key role in neuropathic pain produced by chemotherapeutics (Zhang et al.,2011). The relative contribution of microglial cells versus astrocytes in LPS-induced hyperalgesia is not defined (Guo and Schluesener,2006; Lee et al.,2010).
Minocycline, a second-generation semisynthetic tetracycline antibiotic, also has inhibitory effects on immune and glial cells. Minocycline is a lipid soluble drug that can readily cross the blood-brain barrier (BBB) into the CNS parenchyma (Saivin and Houin,1988). Several studies demonstrated that minocycline possesses anti-inflammatory properties independent of its antimicrobial effects (Stirling et al.,2005). Animal studies indicate minocycline inhibits the production of immune activators by macrophages, microglia, and neurons (Nikodemova et al.,2007a; Zhao et al.,2007; Zink et al.,2005). In vitro, minocycline has an inhibitory effect on the activation, proliferation, and viral replication of microglia, astrocytes macrophages, and lymphocytes (Nikodemova et al.,2007a; Si et al.,2004; Zink et al.,2005). For example, minocycline decreased the activity of microglia and the CNS release of cytokines in experimental allergic encephalitis (Nikodemova et al.,2007a) and blocked the deleterious effects of neuroinflammation on neurogenesis, long-term potentiation, and neuronal survival (Kim et al.,2009; Wang et al.,2004). Recently, interest has arisen on its possible use in the management of chronic pain (Hains and Waxman,2006).
Although several studies have shown that peripheral injection of LPS produces behavioral changes in response to sensory stimuli (Maier et al.,1993), in addition to systemic illness responses, as noted above the glial cell response that drives this phenotype is not established nor is the potential for differential effects of minocycline in abrogating this response. The goal here was to address these gaps in knowledge.
2. Materials and Methods
2.1. Animals
Experiments were performed on male Sprague-Dawley rats (330-380 g; Harlan Laboratories, Indianapolis, IN, USA) housed with free access to food and water and maintained in temperature- and light-controlled rooms (23 ± 2°C, 12/12-hour light/dark cycle with lights on at 07:00) for at least 1 week prior to the study. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to National Institutes of Health guidelines (NIH publication No. 86-23, revised 1985).
2.2. Drug administration and behavioral tests
2.2.1. Drug administration
To examine body temperature and pain behavior, we randomly assigned 26 rats to 1 of 4 treatment group as follows: minocycline/LPS (n = 6), minocycline/vehicle (n = 6), vehicle/LPS (n = 6) and vehicle/vehicle (n = 8). Minocycline (Sigma, CA, USA) was diluted in saline to a concentration of 12.5 mg/ml and buffered by 5 M sodium hydroxide. Rats received an intraperitoneal injection of either the vehicle (saline) or minocycline (25 mg/kg) for 3 consecutive days (day 0, 1 and 2). A repeated injection regimen was selected for minocycline because this was shown necessary to attenuate neuroinflammation in numerous experimental models (Ekdahl et al.,2003; Griffin et al.,2006; Henry et al.,2008a; Nikodemova et al.,2007b; Tomas-Camardiel et al.,2004). LPS (E. coli 055:B5; Sigma) was diluted in sterile normal sterile, and on the third day (at day 2) of the minocycline (or vehicle) injections, unanesthetized rats were injected intraperitoneally with 1 mg/kg LPS or the vehicle alone 30 minutes after minocycline treatment.
2.2.2. Core temperature evaluation
Food was withheld for 2 hours before LPS injection to avoid the thermic effects of digestion. Rectal temperature was measured in conscious, unrestrained rats by gently inserting a small thermistor probe (Vicks V901h-24; Procter & Gamble, Cincinnati, OH, USA) 3 cm into the rectum. Measurements were taken immediately prior to the LPS or vehicle and then at 2, 4, 6, 8, and 24 hours afterward. For all shared time points body temperature was always measured first to minimize potential influences of stress.
2.2.3. Behavioral assessments
Behavioral experiments were conducted in a quiet testing room by an investigator blinded to drug treatment.
2.2.4. Response to thermal stimuli
The hot plate test was used to evaluate thermal nociceptive sensitivity (IITC Life science, Woodland Hills, CA,). Animals were gently placed into an acrylic box with a metal floor that was preheated to 52°C. Paw withdrawal latency was calculated using a timer that was started when the animal was released onto the preheated plate and stopped at the moment of withdrawal, shaking, or licking of either hind paw. Animals were tested at 3, 6, 9, and 24 hours after LPS injections, with 3 trials for each animal at each of these times. Withdrawal threshold for each rat was calculated at the mean of the three trials.
2.2.5. Response to mechanical stimuli
Mechanical withdrawal threshold was determined using an ascending series of von Frey filaments that delivered approximately logarithmic incremental forces (1, 4, 10, 8, 15, 26, and 60 g). Each rat was placed in a clear Plexiglas compartment (25.4 × 25.4 × 10.16 cm; 10 × 10 × 4 in.) on a metal mesh grid. Before each test session, animals were acclimated to the new environment for 10–20 minutes. Each monofilament, starting with the lowest force (1 g), was applied 6 times to the mid-plantar region of the left hind paw of each rat. The monofilament that produced a response of paw withdrawal, flinching, or licking in 3 of the 6 applications was defined as the 50% paw withdrawal threshold (50% PWT). Each animal’s 50% PWT was assessed at 3, 6, 9, and 24 hours after LPS injections.
2.3. Tissue collection
Spinal cord and DRG samples were collected to ascertain cytokine mRNA expression and glial cell activity at 6 hours after LPS injection. In separate groups (n = 6 per group), following deep anesthesia with intraperitoneal injection of pentobarbitone (90 mg/kg body weight; Lundbeck, Deerfield, IL, USA), lumbar spinal cord segments 3-6 and their DRGs were dissected and quickly frozen in liquid nitrogen and stored at −80°C until further processing for real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and western blotting.
2.4. Western blotting
Spinal cord and DRG tissues were homogenized using a Polytron homogenizer (PRO scientific, CT, USA) for 1 minute in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM - glycerophosphate, 1 mM Na3VO4, 1 g/ml leupeptin, and protease and phosphatase inhibitor cocktails (Sigma). Each homogenate was centrifuged for 15 minutes at 13,000 rotations per minute at 4°C to yield supernatant. The protein concentration of the resulting supernatant was determined by Lowry protein assay (Bio-Rad, Hercules, CA, USA). Protein samples (30 g) were heated at 95°C for 5 minutes, separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane using a Transblot SD apparatus (Bio-Rad). After blots had been washed with twice-buffered saline and Tween-20 (TBST; 10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20), the membranes were blocked with 5% skim milk/TBST for 30 minutes and incubated overnight with the primary antibody, followed by a horseradish peroxidase-conjugated appropriate secondary antibody. Primary antibodies included those to the microglial marker ionized calcium-binding adaptor molecule 1 (Iba-1,1:1000; Wako, Osaka, Japan); the astrocyte/satellite cell marker glial fibrillary acidic protein (GFAP, 1:5000, Cell Signaling Technology, Beverly, MA, USA); and macrophage marker, ED-1 (1:1000, AbD Serotec, Raleigh, NC, USA). β-actin was used as the loading control (Sigma, CA, USA). The membranes were then washed, and primary antibodies were detected using enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, UK ).
2.5. Quantitative real-time PCR
Total RNA was isolated from spinal cord and DRGs using TRIzole Reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocol. RNA was then air-dried and re-suspended in RNAse-free pure water and the concentration was measured at 260 nm in a NanoDrop spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA, USA). First-strand cDNA synthesis was performed with random hexamers using a reverse transcription kit (Invitrogen). Quantitative real-time PCR amplification was performed in a final volume of 25 l containing SYBR Green PCR Master Mix (Ambion, Austin, TX, USA) using the Applied Biosystems 7000 sequence detection system (Foster City, CA, USA). The following messages were probed: interleukin-1 beta (IL-1β); interleukin-6 (IL-6); tumor necrosis factor alpha (TNF-α); monocyte chemotactic protein-1 (MCP-1); and RANTES (regulated on activation, normal T expressed and secreted). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer was used as a control. Primer sequences were obtained from GenBank (National Center for Biotechnology Information, www.ncbi.nlm.nih.gov; Table 1). Amplification steps consisted of 50°C for 2 minutes; 95°C for 10 minutes once; then 95°C for 15 seconds, 55°C for 25 seconds, and 72°C for 1 minute for 40 cycles. The threshold cycle (Ct; the number of cycles needed to reach the threshold of detection) was determined for each gene and the relative expression level of each gene was computed using the following formula: relative expression of mRNA = 2−(ΔCtsample−Ctcontrol), where Ctsample is the Ct for the target gene and Ctcontrol is the Ct for GAPDH (for details on the formula, see Applied Biosystems User Bulletin #2).
Table 1.
Primer sequences used for quantitative real-time polymerase chain reaction.
| Genea | Primer sequence (5′–3′) | GenBank accession no. |
|---|---|---|
| IL-1β | GAAGTCAAGACCAAAGTGG (forward) TGAAGTCAACTATGTCCCG (reverse) |
M98820 |
| IL-6 | AAGTTTCTCTCCGCAAGAGACTTCCAG (forward) AGGCAAATTTCCTGGTTATATCCAGTT (reverse) |
NC005013 |
| TNF-α | CTTCAAGGGACAAGGCTG (forward) GAGGCTGACTTTCTCCTG (reverse) |
D00475 |
| MCP-1 | TAGCATCCACGTGCTGTCTC (forward) CCGACTCATTGGGATCATCT (reverse) |
NM031530 |
| RANTES | GCCAACCCAGAGAAGAAGTG (forward) ATCCCCAGCTGGTTAGGACT (reverse) |
NM031116 |
| GAPDH | TGCCAAGTATGATGACATCAAGAAG (forward) AGCCCAGGATGCCCTTTAGT (reverse) |
NM01008 |
TNF-α, tumor necrosis factor alpha; MCP-1, monocyte chemotactic protein-1; IL-1β, interleukin-1 beta; IL-6, interleukin-6; RANTES, regulated on activation, normal T expressed and secreted; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
2.6. Statistical analysis
Results are expressed as means ± standard errors. Data analysis and statistical comparisons were performed using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA, USA). Differences between two groups were assessed using Student’s t-test. For multiple comparisons to analyze the RT-PCR and western blotting or weight loss, analysis of variance (ANOVA) followed by post hoc Dunnett test was performed. Data on pain sensitivity or fever response at the different time points under different treatment conditions were analyzed using 2-way ANOVA followed by post hoc Bonferroni analysis. Differences with p < 0.05 were considered significant.
3. Results
3.1. LPS-induced systemic illness response
3.1.1. Fever
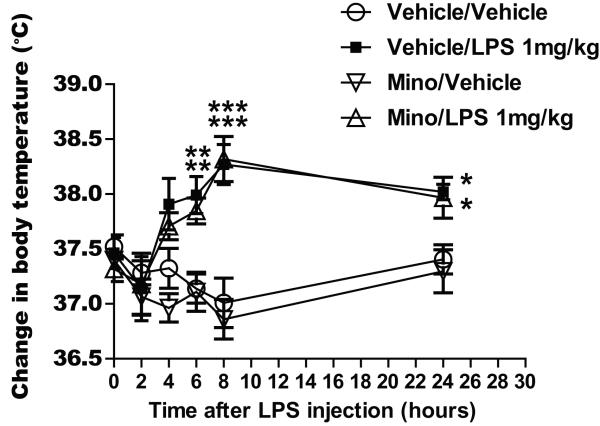
The effects of LPS on body temperature, with and without minocycline treatment, are shown in Figure 1. Rats treated with LPS (vehicle/LPS) showed a significant increase in mean body temperature at 4, 6, 8, and 24 hours after treatment (p < 0.01 at 6 hours; p<0.001 8 hours; p < 0.05 at 24 hours) compared with rats in the vehicle/vehicle control group at these times (treatment group factor: F(3,110) = 9.179, p < 0.001; time factor: F(5,110) = 5.779, p < 0.001).
Fig. 1.
Graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline or vehicle (saline) on change in body temperature following LPS injection. ***p<0.001, **p<0.01 or *p < 0.05 versus the value of the vehicle/vehicle control group at the same time point.
Minocycline did not block LPS-induced fever as these also developed a significant febrile response (Figure 1). Rats in the minocycline/LPS treatment group showed a significant increase in body temperature at 6, 8, and 24 hours after LPS treatment compared with rats in the vehicle/vehicle control group (p < 0.01 at 6 hours; p<0.001 8 hours; p < 0.05 at 24 hours) and this effect was not different from that shown by the LPS treated animals (vehicle/LPS treatment group).
3.2. LPS-induced pain behavior
3.2.1. Thermal stimulus response
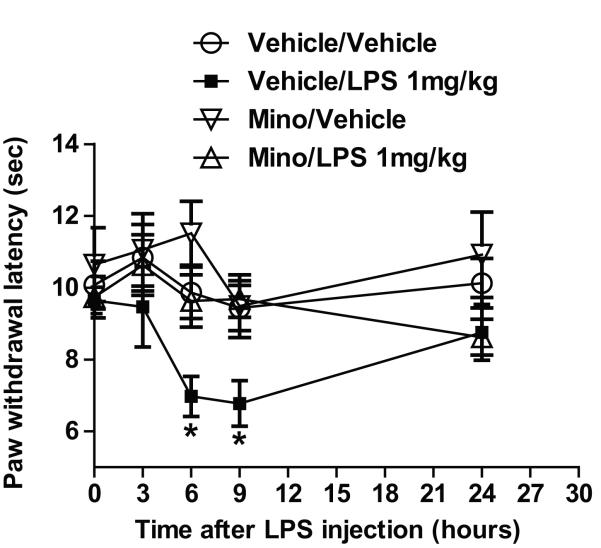
The effects of LPS treatment on the responses to thermal stimuli (paw withdrawal latency), with and without minocycline treatment are shown in Figure 2. In the non-minocycline-treated group, rats with LPS treatment group (vehicle/LPS) showed decreased mean paw withdrawal latency at 6 hours (p < 0.05) and 9 hours (p < 0.05) but not at 24 hours after treatment compared with rats in the vehicle/vehicle control group (treatment group factor: F(3,88) = 1.898; time factor: F(4,88) = 6.584, p < 0.001).
Fig. 2.
Graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline or vehicle (saline) on the paw withdrawal latency (sec) response to thermal stimulation (hot-plate test). *p < 0.05 versus the vehicle/vehicle control group at the same time point.
Minocycline appeared to block the thermal hyperalgesia effect induced by LPS; rats in the minocycline/LPS treatment group did not show a significant decrease in mean paw withdrawal latency compared with rats in the vehicle/vehicle control group.
3.2.2. Mechanical stimulus response
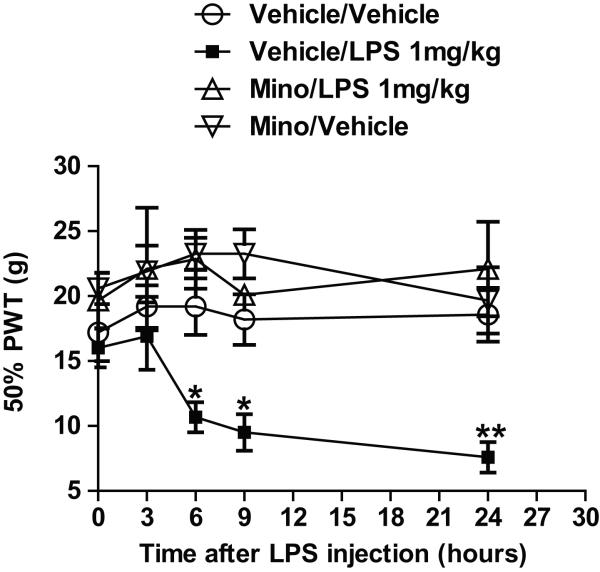
The effects of LPS treatment on response to mechanical stimuli (50% PWT), with and without minocycline treatment, are shown in Figure 3. Though there was some degree of variability, the baseline 50% PWT values were not statistically different between groups (p=0.23). Compared with rats in the vehicle/vehicle control group, rats in the vehicle/LPS treatment group showed reduced mean 50% PWTs at 6, 9, and 24 hours after treatment (p < 0.05 at 6 and 9 hours, p<0.01 at 24hours in LPS; treatment group factor: F(3,88) = 4.417, p < 0.05; time factor: F(4,88) = 5.733, p < 0.001). Maximal reduction in mean 50% PWTs was shown at 24 hours after treatment. Minocycline appeared to block the LPS-induced mechanical hyperalgesia; rats in the minocycline/LPS treatment group did not show significantly different mean 50% PWTs from rats in vehicle/vehicle control group at any measurement time.
Fig. 3.
Graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline or vehicle (saline) on the 50% paw withdrawal threshold (50% PWT; g) response to mechanical stimulation. **p < 0.01 or *p < 0.05 versus the vehicle/vehicle control group at the same time point.
3.3. Iba-1, GFAP and ED-1 expression in spinal cord and DRG
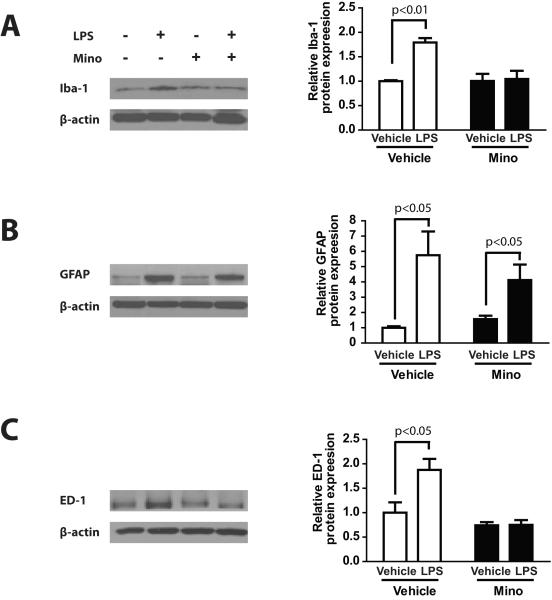
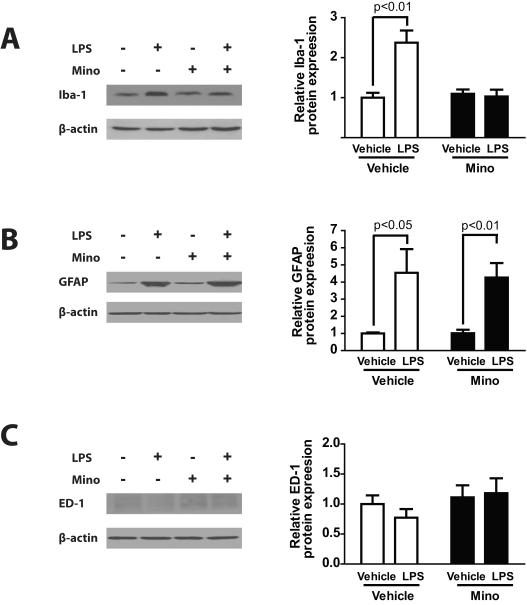
Because the maximal change in behavioral responses occurred at 6 hours after LPS and then gradually decreased from this point, we choose this time point to test glial reactivity and pro-inflammatory responses in the spinal cord and DRG and its modification by minocycline. Western blot data examining glial cell activation by systemic LPS in DRG and spinal cord is shown in Figures 4 and 5. The mean relative Iba-1 expression was significantly different in DRG among each treatment group (Fig.4A, F(3.24) = 12.04, p < 0.001). Post-hoc analysis showed that DRG samples from the LPS treatment group (vehicle/LPS) showed significantly higher mean relative Iba-1 expression than samples from the vehicle/vehicle control group (p < 0.001). Minocycline treatment blocked the effects of LPS treatment as the mean relative expression levels of Iba-1 in samples from the minocycline/LPS treatment group was not statistically different from the mean relative expression levels in samples from the respective minocycline/vehicle treatment group.
Fig. 4.
Western blots and graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline or vehicle (saline) on relative expression levels of (A) ionized calcium-binding adaptor molecule 1 (Iba-1; microglia-like cell marker), (B) glial fibrillary acidic protein (GFAP; satellite cell marker), and (C) ED-1 (macrophage marker) proteins in the dorsal root ganglion (DRG).
Fig. 5.
Western blots and graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline (25 mg/kg; Mino + LPS)or vehicle (saline) on relative expression levels of (A) ionized calcium-binding adaptor molecule 1 (Iba-1; microglia-like cell marker), (B) glial fibrillary acidic protein (GFAP; astrocyte cell marker), and (C) ED-1 (macrophage marker) proteins in the spinal cord.
Mean relative GFAP expression was also higher in samples from vehicle/LPS treatment group compared with relative GFAP expression levels in samples from the vehicle/vehicle control group (Fig.4B, F(3.24) = 5.756, p < 0.01; p < 0.05 for the comparison of vehicle/LPS vs. vehicle/vehicle). In contrast, DRG samples from the minocycline/LPS treated group did not show any significant difference in mean GFAP expression levels compared with samples from the vehicle/LPS group but higher GFAP expression level compared to the vehicle/vehicle and minocycline/vehicle group (p < 0.01), suggesting that minocycline treatment did not affect satellite cell activation.
The mean relative ED-1 expression was higher in DRG samples from the vehicle/LPS treatment group compared with samples from the vehicle/vehicle control group (Fig.4C, F(3.24) = 10.49, p < 0.001; p < 0.01 for the comparison of vehicle/LPS vs. vehicle/vehicle). Minocycline treatment blocked the LPS-induced increase in ED-1 expression levels, suggesting that minocycline also blocked DRG macrophage activation.
When spinal cord samples were analyzed, the Iba-1 expression pattern among the treatment groups was almost the same as in the DRG samples (Fig.5). The vehicle/LPS treatment groups showed higher mean relative spinal cord Iba-1 expression than samples from the vehicle/vehicle control group (Fig.5A, F(3.24) = 12.04, p < 0.001; p < 0.001 for the comparison of vehicle/LPS vs. vehicle/vehicle). Moreover, samples from the minocycline/LPS treated group showed lower mean relative Iba-1 expression levels than samples from the respective vehicle/LPS treatment group (p < 0.001), suggesting that minocycline blocked the effect of LPS on microglial activation in the spinal cord.
Spinal cord samples from the LPS treatment group (vehicle/LPS) showed higher mean relative GFAP expression levels than samples from the vehicle/vehicle control group (Fig.5B, F(3.24) = 5.756, p < 0.01; p < 0.05 for the comparison of vehicle/LPS vs. vehicle/vehicle), and, as in the DRG samples, mean relative GFAP expression levels in the spinal cord samples from minocycline/LPS treated group was not significantly different from those in samples from the vehicle/LPS group. Minocycline/LPS group still showed higher GFAP expression level compared than that of minocycline/vehicle group (p < 0.05), suggesting that minocycline treatment did not affect the activation of spinal astrocytes.
Finally, the mean relative ED-1 expression levels in spinal cord were very weak across all treatment groups indicating very low levels of tissue resident macrophages. This expression levels in spinal cord samples from both minocycline-treated and non-minocycline-treated group with LPS were not significantly different from mean relative expression levels in samples from the vehicle/vehicle control group (Fig.5C, F(3.24) = 0.894), suggesting that LPS failed to provoked macrophage activation in the spinal cord.
3.4. Cytokine mRNA expression
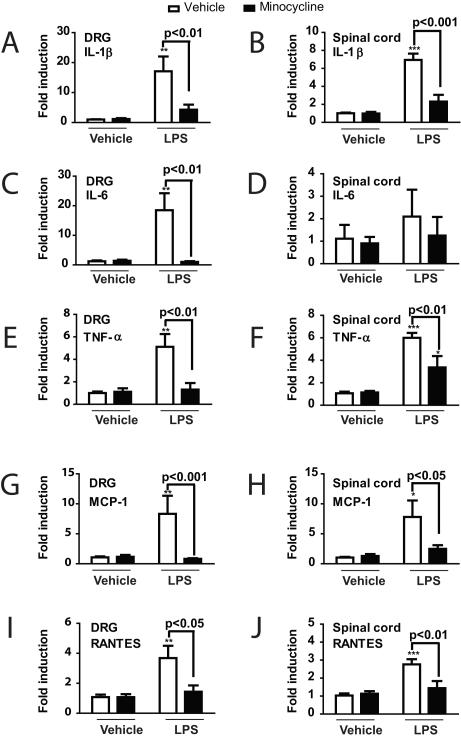
Analysis of cytokine mRNA expression levels in the DRG (Fig. 6A, C, E, G, and I) revealed a significant effect of LPS treatment in increasing the expression of IL-1β, IL-6, TNF-α, MCP-1, and RANTES compared to vehicle/vehicle (F(3,20) = 8.401, p < 0.001; F(3,20) = 8.960, p < 0.001; F(3,20) = 9.095, p < 0.001; F(3,20) = 5.847, p < 0.01; and F(3,20) = 6.948, p < 0.01 respectively). Minocycline treatment prevented this increase as DRG from LPS plus minocycline-treated rats showed significantly lower mean mRNA expression levels for all of tested cytokines compared to the vehicle/LPS group (p < 0.01 for IL-1β, IL-6 and TNF-α; p < 0.001 for MCP-1; p < 0.05 for RANTES). DRG from the minocycline/vehicle group did not show any significant differences in mean relative cytokine mRNA expression levels from those of rats in the vehicle/vehicle control group.
Fig. 6.
Graphs illustrating the effect of lipopolysaccharide (LPS) injection following 3 days of treatment with minocycline or vehicle (saline) on relative mRNA expression levels, expressed as a fold change compared with the control group values for (A, B) interleukin-1 beta (IL-1β), (C, D) interleukin-6 (IL-6), (E, F) tumor necrosis factor alpha (TNF-α), (G, H) monocyte chemotactic protein-1 (MCP-1), and (I, J) RANTES (regulated on activation, normal T expressed and secreted in (A, C, E, G, I) the dorsal root ganglion (DRG) and (B, D, F, H, J) the spinal cord. ***p < 0.001, ** p < 0.01 or *p < 0.05 versus the vehicle/vehicle control group.
In the spinal cord (Fig. 6B, D, F, H, and J), LPS similarly increased mRNA expression levels for all cytokines except IL-6 (F(3,20) = 8.401, p < 0.001 for IL-1β; F(3,20) = 8.960, p < 0.001 for TNF-α; F(3,20) = 9.095, p < 0.001 MCP-1; F(3,20) = 5.847, p < 0.01 for RANTES respectively). Spinal cord samples from minocycline-treated rats (minocycline/LPS group) showed noticeably lower mean mRNA expression levels compared to the vehicle/LPS group across all cytokines measured (p < 0.001 for IL-1β; p < 0.01 for TNF-α and RANTES; p < 0.05 for MCP-1). Spinal cords from the minocycline/vehicle group did not show any significant differences in mean relative cytokine mRNA expression levels from those of rats in the vehicle/vehicle control group.
4. Discussion
The results have shown that LPS treatment produced fever and changes in nociceptive sensitivity that were paralleled by increases in expression markers of glial cells and macrophages in the DRG, glial cells only in the spinal cord, and increased mRNA expression levels for several pro-inflammatory cytokines in both compartments. Minocycline suppressed LPS-induced hyperalgesia and increases in microglial/macrophage cell markers and cytokine expression but did not affect increases in astrocyte markers or LPS-induced increases in core temperature. These results suggest that microglial cells in the spinal cord and DRG are key mediators in sickness, and possibly localized infection, related hyperalgesia through production of proinflammatory cytokines that in turn alter the signaling of primary and spinal neurons in response to cutaneous stimuli.
LPS treatment markedly increased expression of several pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, MCP-1, and RANTES in the DRG and spinal cord. Systemic or perineural administration of numerous pro-inflammatory cytokines, including TNFα, IL-1β and IL-6, induces mechanical hypersensitivity and thermal hyperalgesia (Cunha et al.,1992; Cunha et al.,2000; Cunha et al.,2005; Jin and Gereau,2006; Perkins and Kelly,1994; Safieh-Garabedian et al.,1995; Schäfers et al.,2003a; Schäfers et al.,2003b; Wacnik et al.,2005; Woolf et al.,1997). Increase in the expression of many of these pro-inflammatory cytokines following nerve injury is observed in and around peripheral nerves and in the dorsal root ganglia (Cui et al.,2000; Murphy et al.,1995; Okamoto et al.,2001; Shubayev and Myers,2000; Shubayev and Myers,2001). Peripheral blockade of pro-inflammatory cytokines prevents the development of both inflammatory and neuropathic pain (Cunha et al.,1992; Cunha et al.,2000; Cunha et al.,2005; Safieh-Garabedian et al.,1995; Sommer et al.,1998a; Sommer et al.,1998b; Sommer et al.,2001a; Sommer et al.,2001b; Sorkin and Doom,2000; Woolf et al.,1997). The likely mechanism by which cytokines increase behavioral pain phenotypes is by directly influencing the function of primary afferent neurons. For example, TNFα not only induces transcriptional regulation of down-stream inflammatory mediators, such as induction of IL-1β, IL-6 and Nuclear Factor-B in neurons and Schwann cells (Cunha et al.,2005; Lee et al.,2009; Watkins et al.,1995), but also has a rapid sensitizing effect on primary afferent neurons. TNFα promotes heat-induced CGRP release from nociceptor terminals in skin (Opree and Kress,2000), lowers activation threshold in Aβ- and C-fibers (Junger and Sorkin,2000; Liu et al.,2002; Schäfers et al.,2003a; Zhang et al.,2002), sensitizes transient receptor potential vanilloid receptor 1 (TRPV1) (Nicol et al.,1997) and enhances TTX-resistant sodium currents in primary sensory neurons (Jin and Gereau,2006). In addition to inducing the synthesis of several sensitizers of nociceptors, IL-1β also directly activates peripheral nociceptors. IL-1β acts in a p38 mitogen-activated protein kinase (p38 MAP kinase)-dependent manner, to increase the excitability of primary sensory neurons and increase action potential generation by relieving resting slow inactivation of tetrodotoxin-resistant voltage-gated sodium channels and enhancing persistent TTX-resistant currents (Binshtok et al.,2008). The chemokine MCP-1, also called CCL2, also increases the excitability of primary sensory neurons perhaps acting in an autocrine fashion to increase pain signaling following nerve injury (Jung et al.,2008; Sun et al.,2006; White et al.,2005).
The central nervous system effects of pro-inflammatory cytokines/chemokines have been explored in a more limited fashion. TNFα, IL-1β, IL-6 and MCP-1 are all increased in spinal cord after peripheral nerve injury or inflammation (DeLeo et al.,1997; Holguin et al.,2004; Milligan et al.,2001; Pineau and Lacroix,2007; Sweitzer et al.,2001). Intrathecal application of TNFα, IL-6 or MCP-1 all induce mechanical allodynia and thermal hyperalgesia (Arruda et al.,1998; DeLeo et al.,1996; Gao et al.,2009); whereas in contrast, spinal administration of neutralizing antibodies to TNFα, IL-1β or IL-6 prevents the development of inflammatory and neuropathic pain (Arruda et al.,2000; Choi et al.,2010; Schafers et al.,2001; Schäfers et al.,2003c; Schoeniger-Skinner et al.,2007; Sweitzer et al.,2001). The mechanisms underlying the pronociceptive effects of pro-inflammatory cytokines and chemokines in the spinal cord are largely unknown. TNFα, IL-1β, IL-6 and MCP-1 modulate excitatory and inhibitory synaptic transmission in unidentified dorsal horn neurons (Gao et al.,2009; Kawasaki et al.,2008). Yet, the identity of the specific functional subtypes of neurons that are affected, the receptors and their location, and the second messenger systems involved are not known. Enhancement of excitatory spinal transmission by TNFα has only very recently been shown as dependent upon suppression of on-going inhibitory synaptic transmission (Zhang et al.,2010; Zhang and Dougherty,2011). TNFα is also involved in recruiting Ca2+ permeable AMPA receptors into the plasma membrane of dorsal horn neurons during carrageenan-induced inflammation (Choi et al.,2010). Finally, IL-1β increases the expression of cyclooxygenase-2 in spinal cord dorsal horn during complete Freund’s adjuvant induced paw inflammation (Narita et al.,2008; Samad et al.,2001)
Rats treated with minocycline prior to LPS injections did not demonstrate the LPS-induced hyperalgesia in response to thermal and mechanical stimulation and this appears to have been due to the fact that the normal cytokine response induced by LPS was abrogated. The surprising and important issue that arises based on this observation concerns the relative role of microglia and related cells versus astrocytes in the expression of pain phenotypes. Minocycline is a second-generation semisynthetic tetracycline antibiotic that has unusual anti-inflammatory properties (Dheen et al.,2007). Cell culture and animal experiments have demonstrated that minocycline readily crosses the blood-brain barrier and attenuates microglial cell activation and limits production of inflammatory mediators in a number of conditions (Chen-Roetling et al.,2009; Keilhoff et al.,2011). For instance, minocycline pretreatment of BV-2 (microglia) cultures decreased LPS-stimulated cytokine production (Horvath et al.,2008). Moreover, minocycline blocks impaired working memory that accompanied LPS-induced neuroinflammation (Henry et al.,2008b). In this study, microglia activation was inferred by measurement of increases in Iba-1 protein which is restricted to microglia but not neurons, astrocytes or oligodendrocytes both in vitro and in vivo (Ito et al.,1998). There is increasing evidence that uncontrolled activation of microglia under several pain conditions induces the release of pro-inflammatory cytokines, complement components and other substances leading to the facilitation of pain transmission (Mika,2008). Therefore the results shown here imply that minocycline inhibits spinal microglia release of cytokines that subsequently blocks the LPS-induced pain hyperalgesia.
The results concerning the existence of Iba-1 positive microglia-like immune cells in DRG remain uncertain given that microglia are considered specific CNS resident immune cells. Recently, the presence of Iba-1 positive microglial-like cells in DRG was observed in a model of sciatic nerve transection in rats (Patro et al.,2010). In that study, Iba-1 positive cells were activated following peripheral nerve transection consistent with spinal microglia, which were not co-localized with GFAP (the marker for satellite cells) indicating that Iba-1 positive microglial-like cells are different from satellite cells in DRG. Moreover, all of the Iba-1 positive cells were co-localized with MHC II markers (such as OX-6), characteristic of the microglial phenotype. Although the migration of the peripheral macrophages in DRGs following injury cannot be excluded, Iba-1 positive cells even in the absence of injury supports the presence of resident microglia-like cells in the DRG similar to CNS. Together with previous finding, the data here further suggest that microglia-like immune cells might have a similar role in LPS-induced cytokine release in DRG as spinal cord inducing an increase in neuronal activity by local pro-inflammatory cytokine release.
The increased expression of ED-1 expression in DRG that was significantly increased after LPS injection compared than vehicle group suggests that macrophage infiltration into the DRG is provoked by LPS treatment (Jeon et al.,2011). However, in the spinal cord, ED-1 signal was very weak and was not different from vehicle group. Macrophages enter the DRG after a signal from a peripheral conditioning lesion, and contribute to the pathology of many inflammatory diseases (Schaible et al.,2010). Our study indicates that macrophages infiltration to the DRG might play a key role in production of inflammatory factors that influence nociceptive sensitivity.
Minocycline did not affect the up-regulation of GFAP expression in either the DRG or spinal dorsal horn. GFAP is expressed in satellite cells in the DRG and in astrocytes in the dorsal horn. Because minocycline blocked pain sensitivity caused by LPS, it appears that neither satellite cells in the DRG nor astrocytes in the spinal cord were involved in the LPS-induced changes in nociceptive sensitivity (Cui et al.,2008). Thus, inflammatory, direct nerve injury and infection induced hyperalgesias all appear to include a key microglial but a less important astrocyte component. In contrast, neuropathic pain produced by chemotherapeutics show the opposite, wherein astrocytes play a key role but microglia do not (Zhang et al.,2011; Zheng et al.,2011). Interestingly, minocycline blocks the behavioral phenotype equally well in models of chemotherapy related hyperalgesia as in the nerve injury and LPS models (Boyette-Davis et al.,2011; Boyette-Davis and Dougherty,2011; Zhang et al.,2011). The basis for selectivity of the drug under the differing hyperalgesia conditions is not clear and will likely be of keen interest for further investigation. Moreover, the potential importance of defining differential roles of glial cell subtypes in various specific pain conditions will no doubt also be of interest for further investigation.
Interestingly, minocycline did not affect the LPS-induced fever response. The generation of fever is closely related to the induction of circulating cytokines in blood (Netea et al.,2000). Although not measured here, another study has focused on the effects of minocycline on plasma versus brain levels of interleukins 1 and 6 (Henry et al.,2008a). While, minocycline lowered LPS-induced cytokine levels in hippocampus and cortex there was no effect on the plasma levels. Presumably, the same effect explains the current results with the fever mediated by circulating cytokines that reached the critical regions in the hypothalamus that mediate the pyrogenic response.
In conclusion, systemic LPS induced changes in nociceptive sensitivity, increased glial cell activity and cytokine production. Minocycline blocks the behavioral phenotype by apparently preventing microglial and resident macrophage activation in the dorsal horn and DRG respectively by LPS that result in reduced pro-inflammatory cytokine expression. A lack of effect of astrocytes in the LPS model may suggest differential glial cell involvement in particular models of hyperalgesia.
Research highlights.
LPS hyperalgesia involves activation of microglial/macrophage activity in DRG and spinal cord.
LPS induced hyperalgesia is inhibited by minocycline.
Minocycline suppresses activation of microglia/macrophages in DRG and spinal cord but does not affect astrocytes.
Table 2.
The effect of minocycline alone on core temperature, thermal and mechanical withdrawal threshold.
| Day 0 (prior to i.p. minocycline) |
Day 2 (after i.p. minocycline) |
|||
|---|---|---|---|---|
| Measure | Vehicle | Minocycline | Vehicle | Minocycline |
| Core temperature (°C) | 37.56 ± 0.08 | 37.58 ± 0.06 | 37.50 ± 0.08 | 37.46 ± 0.07 |
| Thermal threshold (sec) | 9.13 ± 0.50 | 10.11 ± 0.71 | 9.53 ± 0.52 | 10.19 ± 0.58 |
| Mechanical threshold (g) | 17.64 ± 1.59 | 19.17 ± 1.17 | 17.82 ± 1.42 | 20.13 ± 1.17 |
Either the vehicle (saline, N=14) or minocycline (25 mg/kg, N=12) was treated for 3 consecutive days (Day 0, 1 and 2, once a day). On Day 0 (before minocycline injection) and Day 2 (after minocycline injection), core temperature and withdrawal thresholds were measured to examine the effect of minocycline itself prior to LPS injection. Data were expressed by means ± SEM.
Acknowledgments
This work was supported by National Institutes of Health grant NS466606, National Cancer Institute grant CA124787, and MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arruda JL, Colburn RW, Rickman AJ, Rutkowski MD, DeLeo JA. Increase of interleukin-6 mRNA in the spinal cord following peripheral nerve injury in the rat: potential role of IL-6 in neuropathic pain. Brain Res. Mol. Brain Res. 1998;62:228–235. doi: 10.1016/s0169-328x(98)00257-5. [DOI] [PubMed] [Google Scholar]
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA. Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. doi: 10.1016/s0006-8993(00)02807-9. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors Are Interleukin-1 beta Sensors. J. Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp. Neurol. 2011;229:353–357. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152:308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem. Biophys. Res. Commun. 2009;386:322–326. doi: 10.1016/j.bbrc.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010 doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J-G, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav. Immun. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The Pivotal Role of Tumor-Necrosis-Factor-Alpha in the Development of Inflammatory Hyperalgesia. British Journal of Pharmacology. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br. J. Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr., Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Ann. N. Y. Acad. Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Colburn RW, Nichols M, Malhotra A. Interleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy model. J. Interferon Cytokine Res. 1996;16:695–700. doi: 10.1089/jir.1996.16.695. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Colburn RW, Rickman AJ. Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res. 1997;759:50–57. doi: 10.1016/s0006-8993(97)00209-6. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J. Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Guo L-H, Schluesener HJ. Acute but not chronic stimulation of glial cells in rat spinal cord by systemic injection of lipopolysaccharide is associated with hyperalgesia. Acta Neuropathol. 2006;112:703–713. doi: 10.1007/s00401-006-0135-z. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008a;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008b;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin A, O’Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Nutile-McMenemy N, Alkaitis MS, DeLeo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J. Neurochem. 2008;107:557–569. doi: 10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Sung JK, Cho HJ. Expression of monocyte chemoattractant protein-1 and its induction by tumor necrosis factor receptor 1 in sensory neurons in the ventral rhizotomy model of neuropathic pain. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Jin XC, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Schweizer H, John R, Langnaese K, Ebmeyer U. Minocycline neuroprotection in a rat model of asphyxial cardiac arrest is limited. Resuscitation. 2011;82:341–349. doi: 10.1016/j.resuscitation.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Kim MJ, Park JM, Lee SH, Kim YJ, Ryu S, Kim YH, Yoon BW. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J. Neurol. Sci. 2009;279:70–75. doi: 10.1016/j.jns.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Lee KM, Jeon SM, Cho HJ. Tumor necrosis factor receptor 1 induces interleukin-6 upregulation through NF-kappaB in a rat neuropathic pain model. Eur. J. Pain. 2009;13:794–806. doi: 10.1016/j.ejpain.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhao YQ, Ribeiro-Da-Silva A, Zhang J. Distinctive response of CNS glial cells in oro-facial pain associated with injury, infection and inflammation. Mol. Pain. 2010;6:79. doi: 10.1186/1744-8069-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Li H, Brull SJ, Zhang J-M. Increased sensitivity of sensory neurons to tumor necrosis factor in rats with chronic compression of the lumbar ganglia. J. Neurophysiol. 2002;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol. Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RPA, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J. Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Shimamura M, Imai S, Kubota C, Yajima Y, Takagi T, Shiokawa M, Inoue T, Suzuki M, Suzuki T. Role of interleukin-1 beta and tumor necrosis factor-alpha-dependent expression of cyclooxygenase-2 mRNA in thermal hyperalgesia induced by chronic inflammation in mice. Neuroscience. 2008;152:477–486. doi: 10.1016/j.neuroscience.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin. Infect. Dis. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J. Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J. Biol. Chem. 2007a;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J. Biol. Chem. 2007b;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp. Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor- , IL-1β, and IL-6 but not IL-8 in the development of heat hyperalgesia: Effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro N, Nagayach A, Patro IK. Iba1 expressing microglia in the dorsal root ganglia become activated following peripheral nerve injury in rats. Indian J. Exp. Biol. 2010;48:110–116. [PubMed] [Google Scholar]
- Perkins MN, Kelly D. Interleukin-1-Beta Induced Desarg(9)Bradykinin-Mediated Thermal Hyperalgesia in the Rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre J, Woolf CJ. Interleukin-1 -mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schafers M, Brinkhoff J, Neukirchen S, Marziniak M, Sommer C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci. Lett. 2001;310:113–116. doi: 10.1016/s0304-3940(01)02077-8. [DOI] [PubMed] [Google Scholar]
- Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J. Neurosci. 2003a;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003b;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor- induces mechanical allodynia after spinal nerve ligation by activation of p38 MIAPK in primary sensory neurons. J. Neurosci. 2003c;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Von Banchet GS, Boettger MIK, Brauer R, Gajda Ml, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. N. Y. Acad. Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- Schoeniger-Skinner DK, Ledeboer A, Frank MG, Mlilligan ED, Poole S, Martin D, Mlaier SF, Watkins LR. Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120. Brain Behav. Immun. 2007;21:660–667. doi: 10.1016/j.bbi.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNFα receptors. J. Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- Si Q, Cosenza M, Kim MO, Zhao ML, Brownlee M, Goldstein H, Lee S. A novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microglia. J. Neurovirol. 2004;10:284–292. doi: 10.1080/13550280490499533. [DOI] [PubMed] [Google Scholar]
- Sommer C, Lindenlaub T, Teuteberg P, Schafers M, Hartung T, Toyka KV. Anti-TNF-antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001a;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- Sommer C, Marziniak M, Myers RR. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998a;74:83–91. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafer M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J. Peripher. Nerv. Syst. 2001b;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNFα receptor 1. Exp. Neurol. 1998b;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM. Epineurial application of TNFα elicits an acute mechanical hyperalgesia in the awake rat. J. Peripher. Nerv. Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J. Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol. Dis. 2004;16:190–201. doi: 10.1016/j.nbd.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Simone DA, Wilcox GL, Beitz AJ. Nociceptive characteristics of tumor necrosis factor-alpha in naive and tumor-bearing mice. Neuroscience. 2005;132:479–491. doi: 10.1016/j.neuroscience.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Wang Q, Rowan MJ, Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J. Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Brewer MT, Relton J, Maier SF. Mechanisms of tumor necrosis factor- (TNF-α) hyperalgesia. Brain Res. 1995;692:244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann. Intern. Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumor necrosis factor. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dougherty PM. Acute Inhibition of Signaling Phenotype of Spinal GABAergic Neurons by Tumour Necrosis Factor-{alpha} J. Physiol. 2011 doi: 10.1113/jphysiol.2011.215301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J. Neurosci. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yoon S-Y, Zhang H, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J. Pain. 2011 doi: 10.1016/j.jpain.2011.12.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, Li H, Liu B, Brull SJ. Acute topical application of tumor necrosis factor evokes protein kinase A-dependent responses in rat sensory neurons. J. Neurophysiol. 2002;88:1387–1392. doi: 10.1152/jn.2002.88.3.1387. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol. Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 2011;176:447454. doi: 10.1016/j.neuroscience.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]