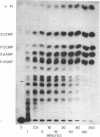
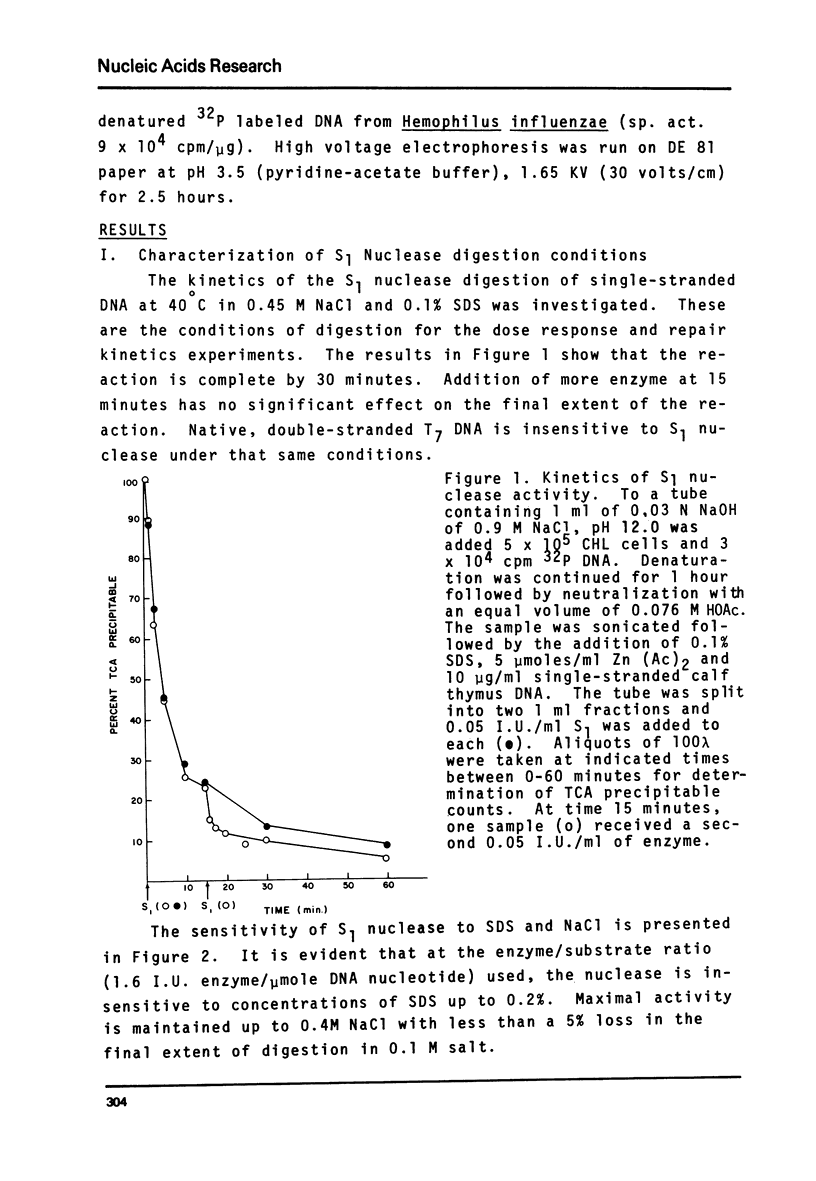
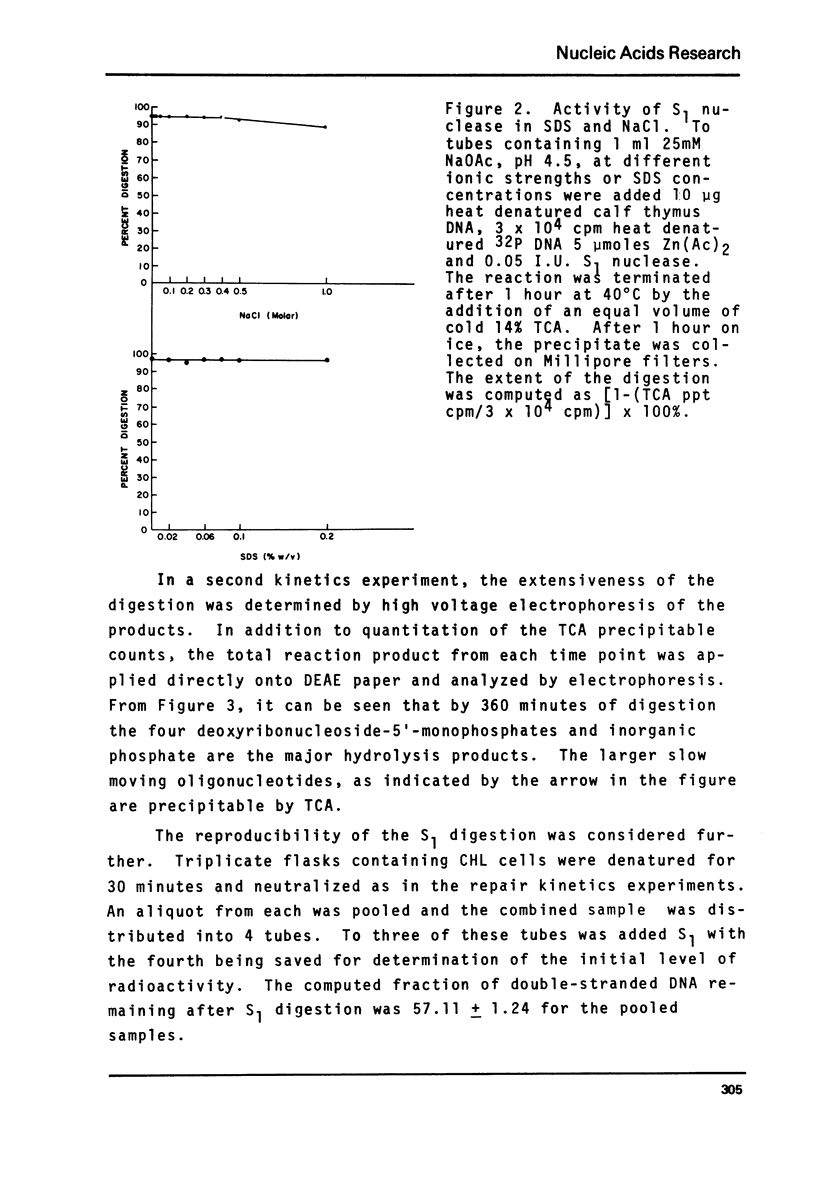
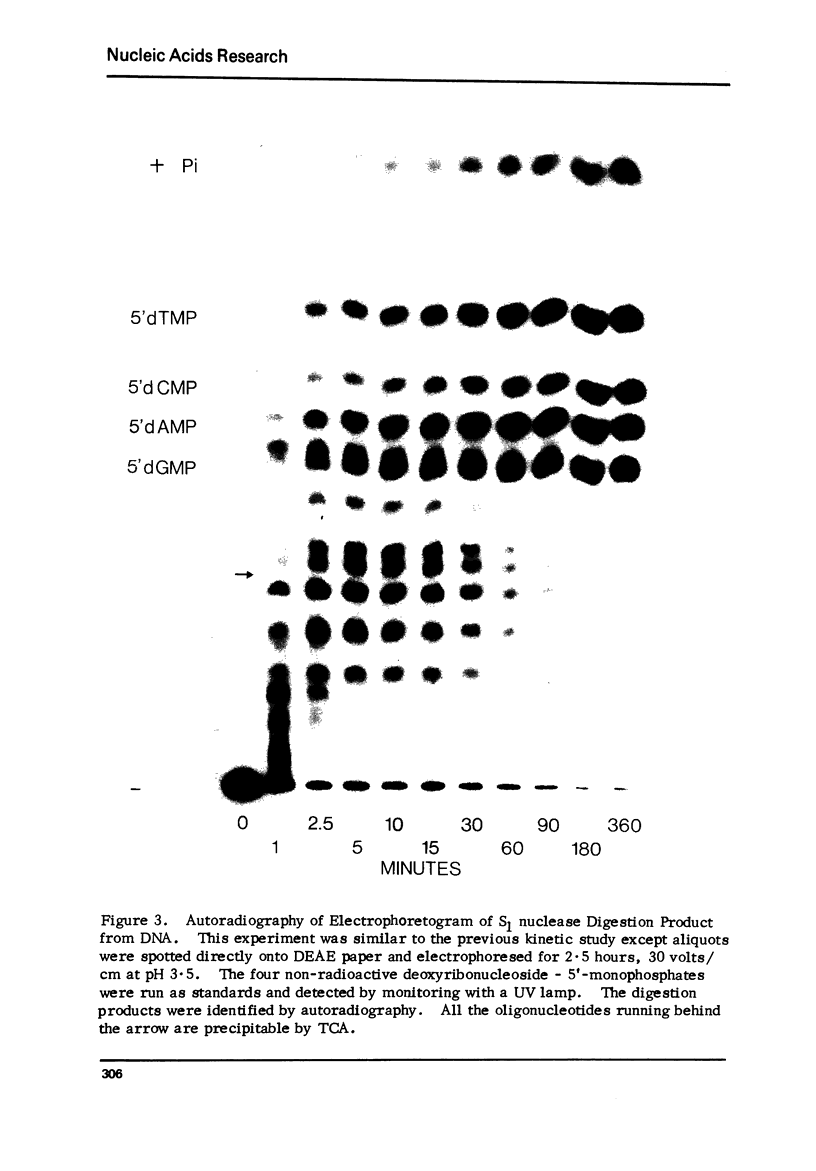
Abstract
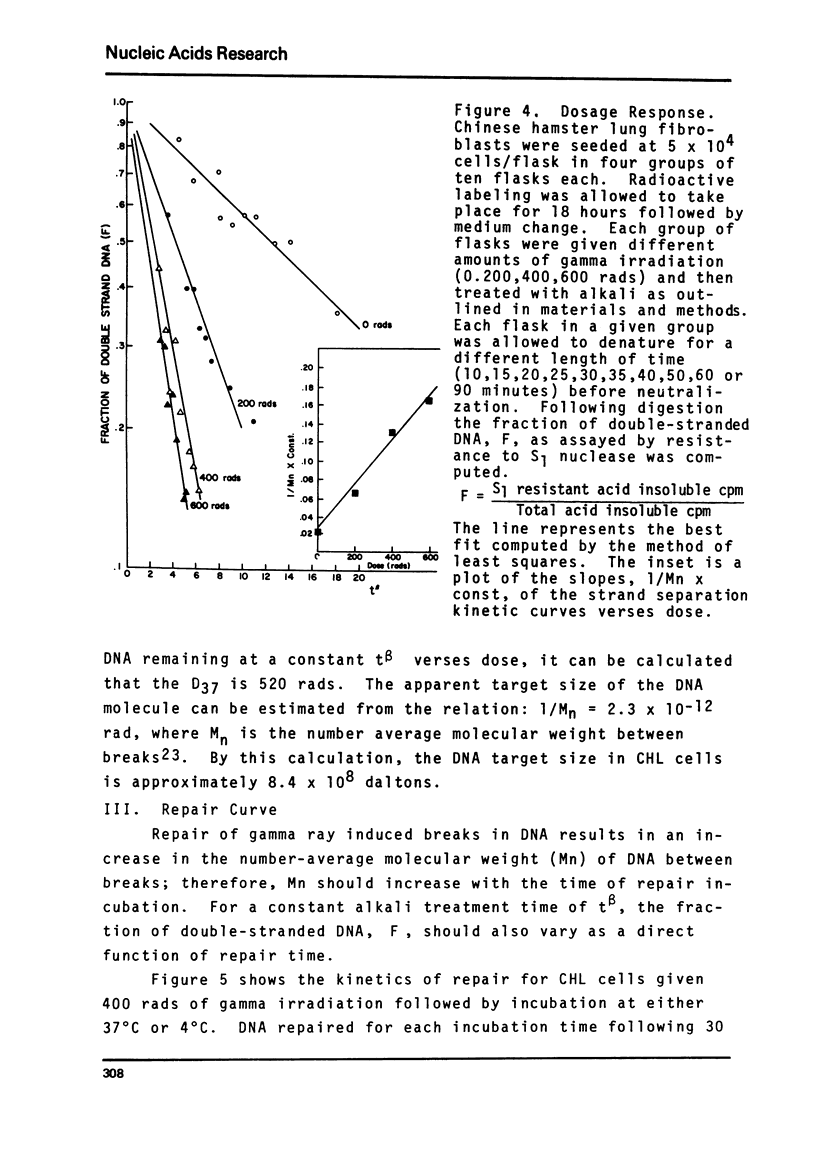
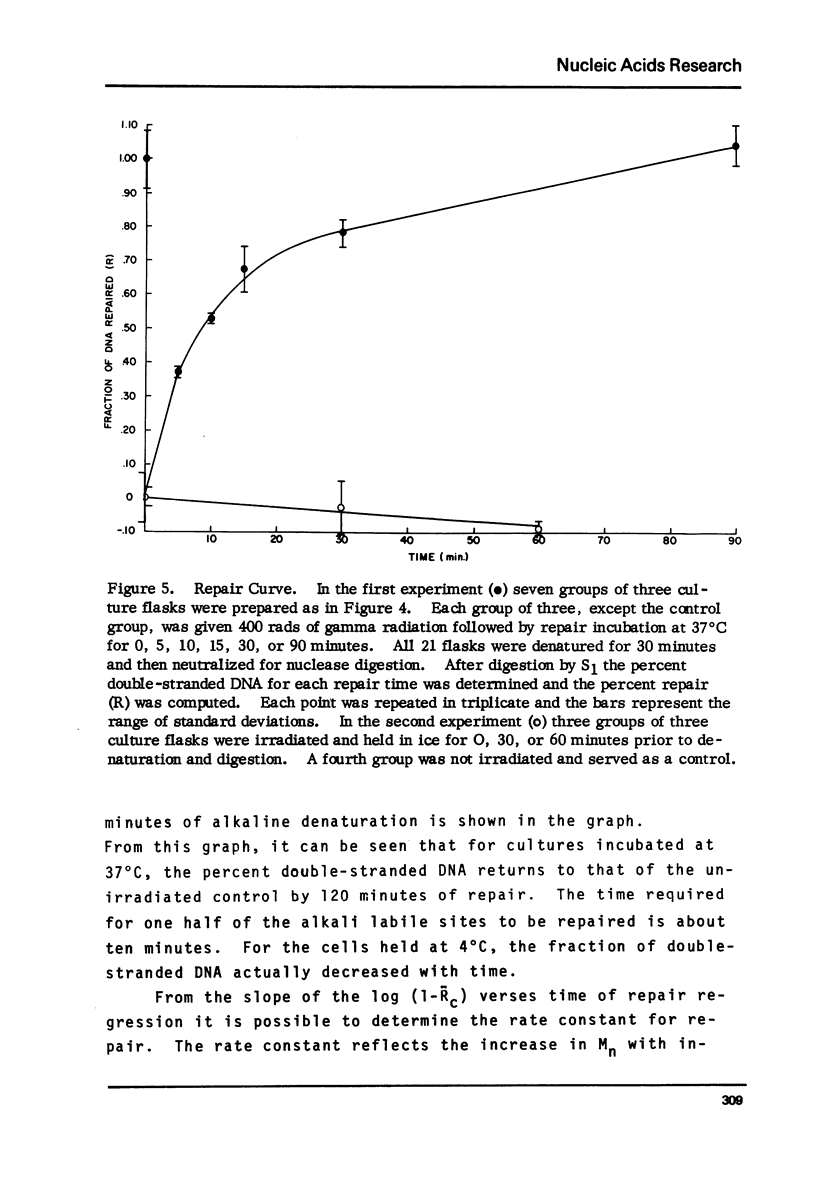
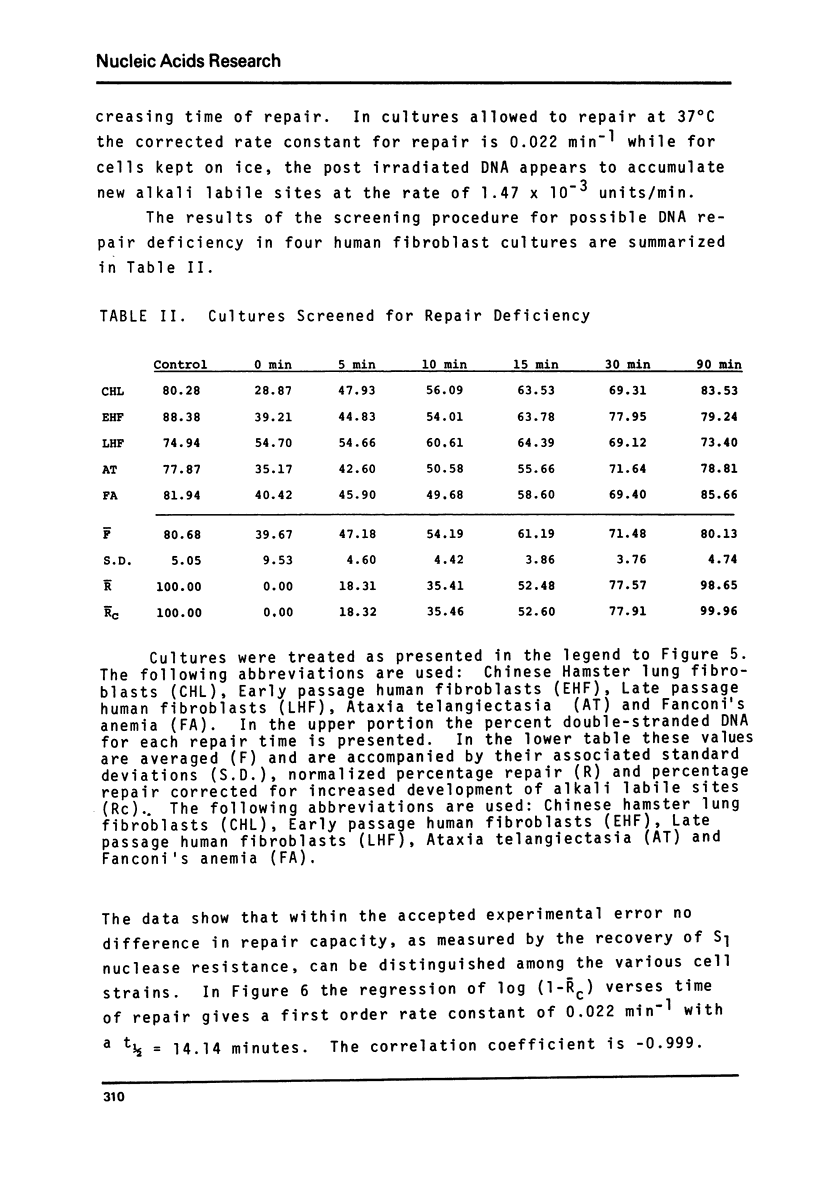
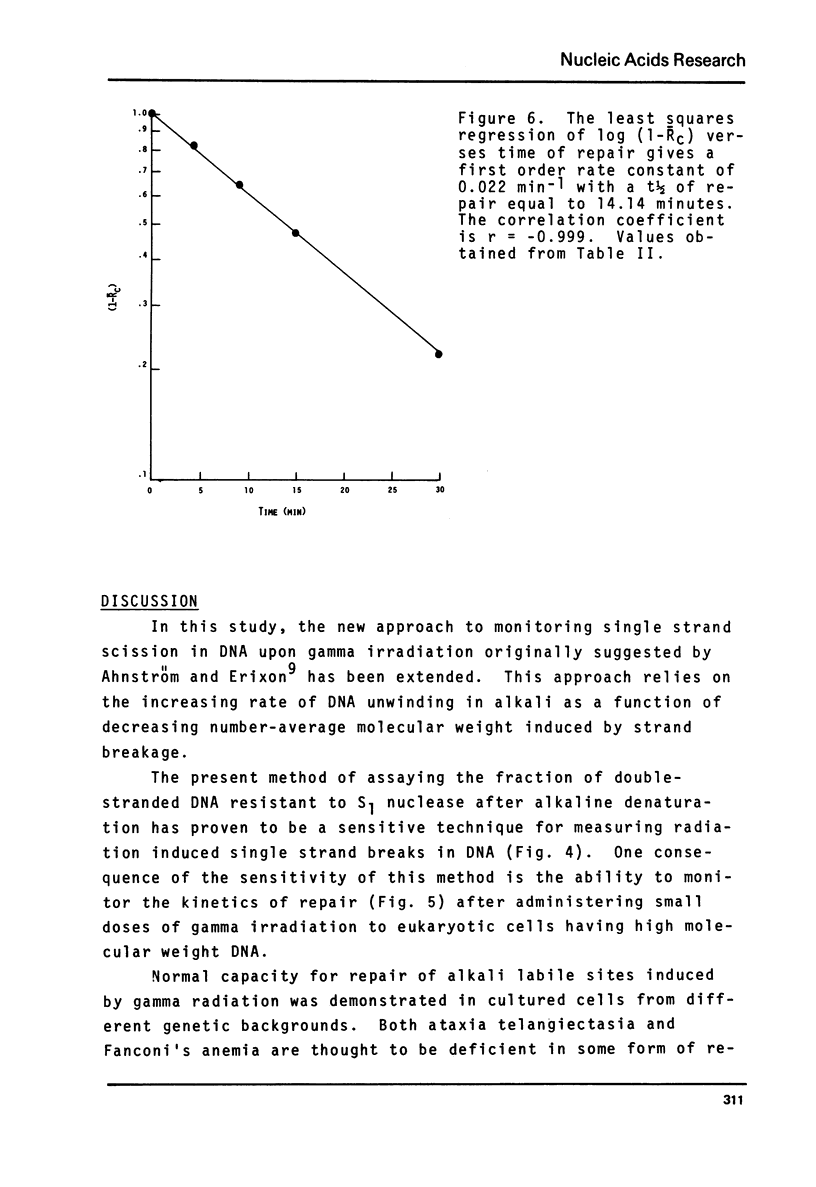
A sensitive new approach for measuring the repair of single strand breaks in DNA induced by low doses of gamma irradiation was tested in cultured fibroblasts from Chinese hamster lung, human afflicted with ataxia telangiectasia or Fanconi's anemia and in normal cells of early and late passages. The assay is based on the increasing rate of strand separation of DNA duplexes in alkali for molecules with increasing numbers of single strand scissions. DNA strand separation is shown to follow the relation, in F = -(1/Mn - const) - tbeta where F is the proportion of double-stranded DNA, detected as S1 nuclease resistant, after alkaline denaturation time, t. Mn is the number-average molecular weight of DNA between single strand breaks. beta less than 1 is an empirically determined constant. The results suggest an increase in the number-average molecular weight between breaks, Mn, with increasing times for repair. The final level attained corresponds to the Mn of control DNA in unirradiated cells. As few as one break introduced into 109 daltons of single-stranded control cell DNA can be detected. The kinetics, requirements and sensitivities of this assay are described.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnström G., Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- Bedford J. S., Mitchell J. B., Griggs H. G., Bender M. A. Cell killing by gamma rays and beta particles from tritiated water and incorporated tritiated thymidine. Radiat Res. 1975 Sep;63(3):531–543. [PubMed] [Google Scholar]
- Clark C. C., Kefalides N. A. Carbohydrate moieties of procollagen: incorporation of isotopically labeled mannose and glucosamine into propeptides of procollagen secreted by matrix-free chick embryo tendon cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):34–38. doi: 10.1073/pnas.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind M. M., Kamper C. Two forms of repair of DNA in mammalian cells following irradiation. Biophys J. 1970 Mar;10(3):237–245. doi: 10.1016/S0006-3495(70)86296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind M. M. Sedimentation of DNA released from Chinese hamster cells. Biophys J. 1971 Jun;11(6):502–520. doi: 10.1016/S0006-3495(71)86231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn K. W., Friedman C. A., Ewig R. A., Iqbal Z. M. DNA chain growth during replication of asynchronous L1210 cells. Alkaline elution of large DNA segments from cells lysed on filters. Biochemistry. 1974 Sep 24;13(20):4134–4139. doi: 10.1021/bi00717a011. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Ormerod M. G. Double-strand breaks in the DNA of a mammalian cell after x-irradiation. Biochim Biophys Acta. 1970 Oct 15;217(2):268–277. doi: 10.1016/0005-2787(70)90526-5. [DOI] [PubMed] [Google Scholar]
- Lett J. T., Caldwell I., Dean C. J., Alexander P. Rejoining of x-ray induced breaks in the DNA of leukaemia cells. Nature. 1967 May 20;214(5090):790–792. doi: 10.1038/214790a0. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Poon P. K., O'Brien R. L., Parker J. W. Defective DNA repair in Fanconi's anaemia. Nature. 1974 Jul 19;250(463):223–225. doi: 10.1038/250223a0. [DOI] [PubMed] [Google Scholar]
- Rydberg B. The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat Res. 1975 Feb;61(2):274–287. [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Simpson J. R., Nagle W. A., Bick M. D., Belli J. A. Molecular nature of mammalian cell DNA in alkaline sucrose gradients. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3660–3664. doi: 10.1073/pnas.70.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Tägder K., Scheuermann W. Estimation of absorbed doses in the cell nucleus after incorporation of 3H- or 14C-labeled thymidine. Radiat Res. 1970 Jan;41(1):202–216. [PubMed] [Google Scholar]
- Vincent R. A., Jr, Sheridan R. B., 3rd, Huang P. C. DNA strained breakage repair in ataxia telangiectasia fibroblast-like cells. Mutat Res. 1975 Dec;33(2-3):357–366. doi: 10.1016/0027-5107(75)90211-0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]