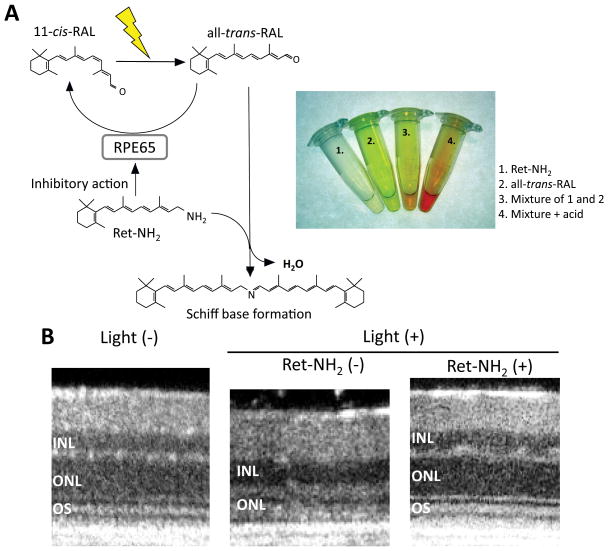
Figure 5. Pharmacological innovation to protect photodamage mediated by all-trans-RAL.
Accumulation of all-trans-RAL is prevented by two pharmacological actions of retinylamine (Ret-NH2). First, free all-trans-RAL is neutralized by Schiff base formation between all-trans-RAL and Ret-NH2 (A). This chemical reaction can be monitored in a color change in the reaction-mixture. Second, free all-trans-RAL generation can be decreased by the inhibitory action of Ret-NH2 in the visual cycle. Specific-binding of Ret-NH2 to RPE65 can prevent an isomerization reaction and slow down regeneration of 11-cis-RAL, which can consequently decrease free all-trans-RAL during light exposure. These two pharmacological actions can protect Rdh8−/−Abca4−/−retina from photodamage (B). Representative in vivo retinal images by high-definition SD-OCT clearly reveal that Rdh8−/−Abca4−/− mice treated with Ret-NH2 can maintain normal morphology of the retina whereas outer nuclear layer (ONL) are severely degenerated in vehicle treated mice. INL, inner nuclear layer; ONL, outer nuclear layer; OS, outer segment.