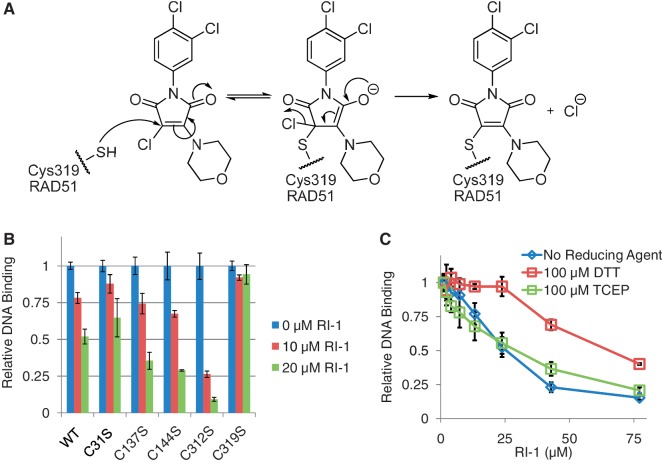
Figure 3.
RI-1 directly binds to human RAD51 at cysteine-319. (A) A chemical mechanism is proposed, by which RI-1 binds a cysteine residue on HsRAD51. (B) Purified HsRAD51 proteins (at 0.2 µM) containing cysteine to serine mutations were tested for ability to bind ssDNA, in the presence or absence of RI-1. Relative DNA binding is reported as a function of fluorescence polarization as described in the ‘Materials and Methods’ section, normalized to the 0 µM RI-1 condition of each protein. (C) The ability of RI-1 to inhibit the ssDNA binding activity of WT HsRAD51 protein (0.25 µM) was evaluated in the presence of different reducing agents, including dithiothreitol (DTT) and tris(2-carboxyethyl)phosphine (TCEP).