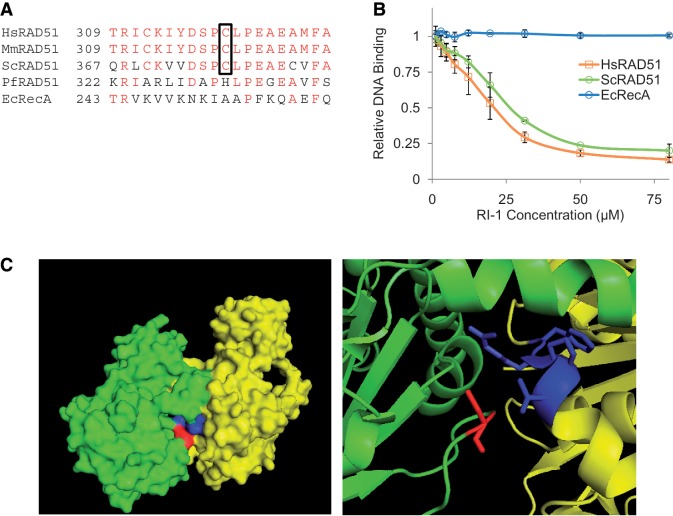
Figure 4.
The cysteine binding target of RI-1 is highly conserved among eukaryotic RAD51 proteins and is located in an interface used for protein–protein interactions. (A) A sequence alignment is shown for homologous recombinase proteins of various species. Amino acids with identity are displayed in red, and the cysteine corresponding to C319 in HsRAD51 is boxed. (B) The ability of RI-1 to inhibit the ssDNA binding activity of recombinase proteins from various species (0.35 µM HsRAD51, 0.42 µM ScRAD51, 0.30 µM EcRecA) was evaluated. Relative DNA binding is reported as a function of fluorescence polarization as described in the ‘Materials and Methods’ section. (C) The crystal structure (previously solved and described by Conway et al.) is shown for two adjacent monomers (one monomer displayed in green and one in yellow) of ScRAD51 as a surface rendering (left) and a magnified cartoon (right). The cysteine binding target of RI-1 is displayed in red (within the green monomer) and the α9 chain of the opposing monomer is displayed in blue (within the yellow monomer).