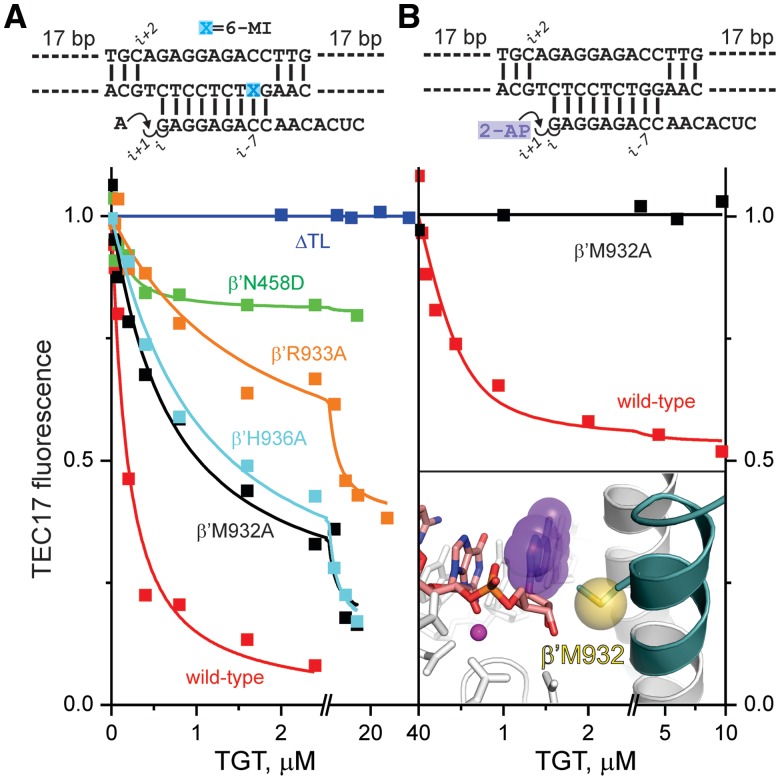
Figure 5.
The effects of amino acid substitutions in RNAP on translocation equilibrium. Schematics of the initial scaffolds are indicated above the graphs. (A) TGT titration of AMP-extended TECs assembled using wild-type (red), ΔTL (blue), β′N458D (green), β'R933A (orange), β′H936A (cyan) and β′M932A (black) RNAPs. (B) TGT titration of 2-AP ribonucleoside monophosphate-extended TECs assembled using wild-type and β′M932A RNAPs. Inset: Upon TL folding (dark teal helix) and backward translocation, the sulfur atom of β′Met932 (yellow sphere) closely (∼5 Å) approaches the 2-AP nucleobase (violet) at the RNA 3′ end, thereby quenching its fluorescence. Active-site Mg2+ is depicted as a magenta sphere. Catalytic aspartates and β′Asn458 are shown as sticks. The representation is drawn using the model of the pre-translocated TEC of T. thermophilus RNAP (see Supplementary Figure S4A); residue numbers correspond to E. coli RNAP.