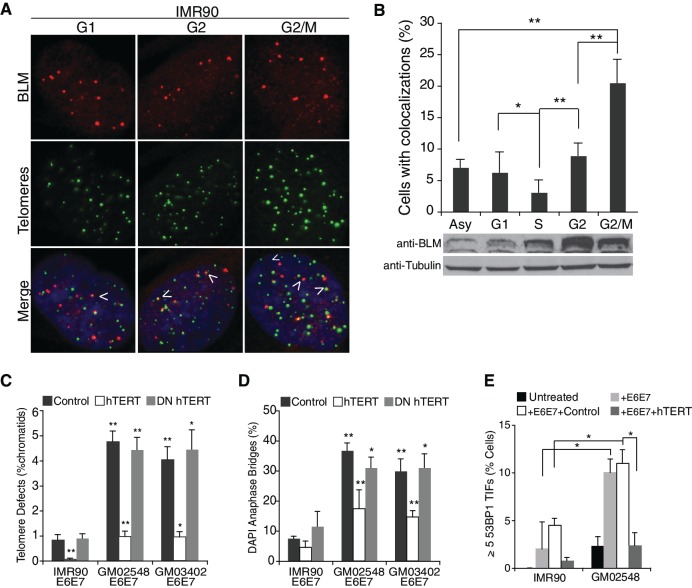
Figure 1.
BLM localizes to a subset of telomeres preferentially in G2/M. (A) IF-FISH images of synchronized IMR90 cells, using a BLM specific antibody (red) and a telomeric probe, FITC-[CCCTAA]4 (green). Arrows indicate colocalization events between BLM and telomeric foci. (B) Top panel: quantification of IF-FISH data, showing the percentage of IMR90 cells with over two colocalization events. At least 100 cells were counted per time-point and values were averaged from four independent experiments. Error bars represent the standard deviation and P-values were calculated with a Student’s t-test (*P < 0.05 and **P < 0.005). Bottom panel: western blot of synchronized IMR90 cells probed with BLM antibody or a tubulin control. IMR90 lysates were collected at the indicated stages of the cell cycle. (C) Percentage of chromatids with TDs in IMR90 or BS fibroblast cell lines expressing an empty vector control, hTERT or DN_hTERT. At least 1500 chromatids per cell line, per experiment were counted from three independent experiments. (D) DAPI-positive anaphase bridges in IMR90 and BS fibroblast cells expressing an empty vector control, hTERT or DN_hTERT. At least 55 anaphases per cell line were counted from three independent experiments. (E) Percentage of IMR90 and GM02548 BS cells with over five TIF. Cells were fixed with 4% PFA and prepared for IF-FISH with a 53BP1-specific antibody and the telomeric probe FITC-[CCCTAA]4. At least 100 cells from each cell line were counted in two independent experiments. Error bars represent the standard deviation and P-values were calculated with a Student’s t-test (*P < 0.05 and **P < 0.005).