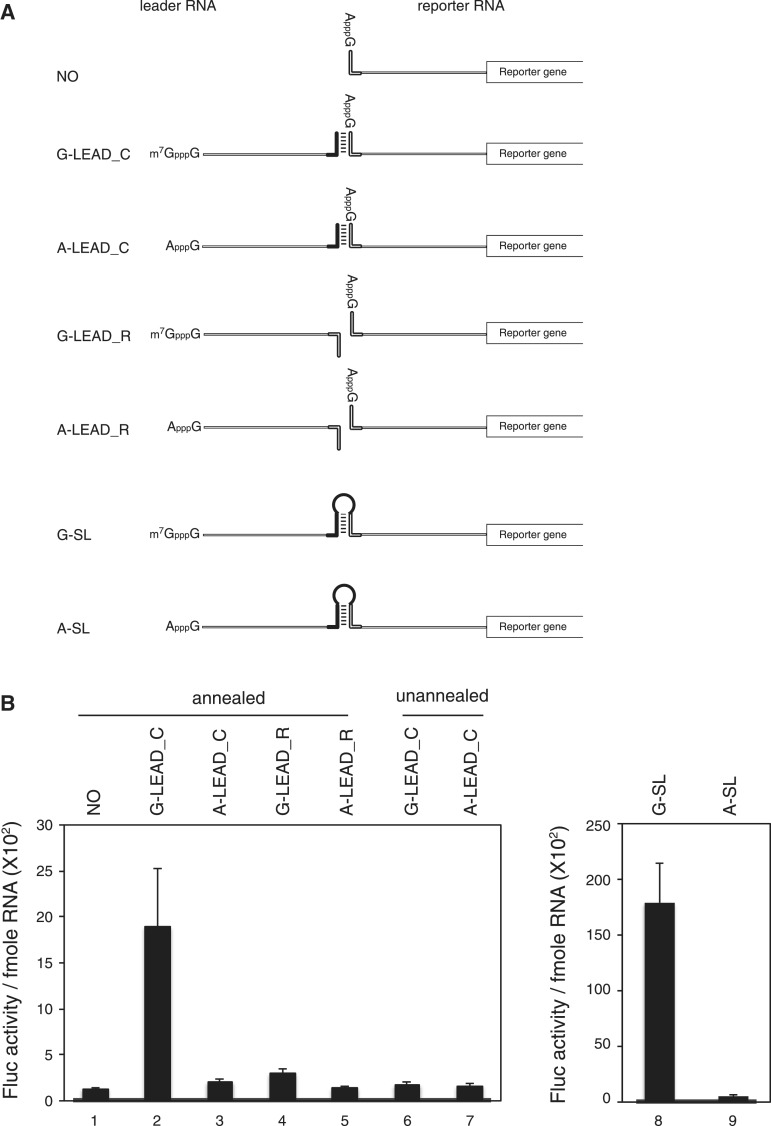
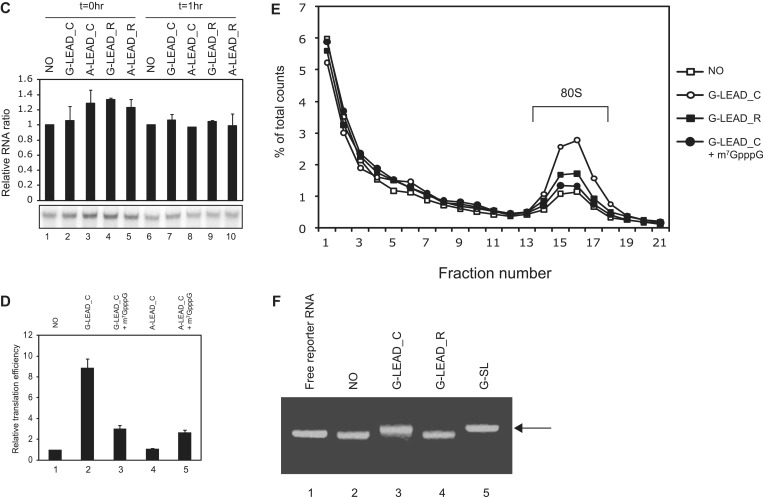
Figure 1.
Enhancement of translation by an m7GpppG-capped (G-capped) leader RNA associated with a reporter RNA via a dsRNA bridge. (A) Schematic diagram of the leader and reporter RNAs. The reporter RNA contains a reporter gene (firefly luciferase). The leader RNAs contain 3′ sequences that are the complements (G-LEAD_C and A-LEAD_C, solid bar) or reverse complements (G-LEAD_R and A-LEAD_R, open bar) of 5′ sequences in the reporter RNAs. The leader RNAs are either m7GpppG-capped (G-LEAD_C and G-LEAD_R) or ApppG-capped (A-LEAD_C and A-LEAD_R). RNA complexes containing both leader and reporter RNAs covalently connected by a stem-loop structure, thus within a single molecule, and with an m7GpppG-cap (G-SL) or an ApppG-cap (A-SL), were constructed to serve as control mRNAs for experiments involving G-LEAD_C and A-LEAD_C, respectively. (B) Reporter translational efficiencies were determined by measuring firefly luciferase activity after pre-annealing of leader and reporter RNAs (lanes 1–5), or with omission of the pre-annealing step (lanes 6 and 7). The luciferase activity in each translation mixture was monitored after 1 h incubation. The translation efficiencies of G-SL and A-SL were also measured and depicted in a separate graph. Similar relative translation efficiencies of the reporter and lead RNA combinations were observed from translation reactions for 30 min instead of 1 h (Supplementary Figure S4). The error bars indicate the standard deviations of nine independent experiments. (C) Leader RNAs did not affect the stability of reporter RNA in translation mixtures. Such mixtures, containing leader and radiolabelled reporter RNAs, were incubated on ice (lanes 1–5; t = 0 h) or subjected to in vitro translation at 30°C for 1 h (lanes 6–10, t = 1 h). (D) The effect of a cap analogue (0.2 mM) on cap-dependent translation mediated by capped leaders associated with reporter RNAs through dsRNA bridges was analyzed relative to the luciferase activity from a reporter RNA lacking a leader RNA (lane 1). (E) Effects of various leader RNAs on 80 S complex formation on reporter RNAs. The amounts of 80 S complex on reporter RNAs labelled with [32P] were monitored by sucrose density gradient analysis after incubation of leader and reporter RNAs in RRL for 15 min in the presence of cycloheximide. (F) Confirmation of hybridization between leader RNAs and reporter RNAs. Annealing reactions with reporter RNA only (NO), or with mixtures of leader RNAs (G-LEAD_C and G-LEAD_R) and reporter RNA, were performed using a thermocycler (lanes 2–4). Reporter RNA was used without an annealing step (lane 1), and the reporter RNA G-SL, which contains covalently linked leader RNA (lane 5), was used as controls. The relevant reporter and leader RNAs are shown in panel A. RNAs were resolved by electrophoresis in a 1% (w/v) agarose gel. Most reporter RNAs became annealed with complementary leader RNAs (G-LEAD_C), as indicated by arrows (lane 3). Reporter RNAs mixed with leader RNAs lacking complementary sequences remained in monomer form (lane 4).