Abstract
Background
The recurrence of regurgitation following surgical mitral valve (MV) repair remains a significant clinical problem. Mitral annuloplasty rings are commonly used in MV repair procedures. The purpose of this study was to demonstrate the feasibility of transvenous valve-in-ring (VIR) implantation using the Melody® valve, which is a valved-stent designed for percutaneous pulmonary valve replacement, and 4 distinct types of annuloplasty ring (AR) in an ovine model.
Methods
Ten sheep underwent surgical MV annuloplasty ring placement (N=10: CE-Physio [N=5]; partial ring [N=3]; flexible ring [N=1]; saddle ring [N=1]). All animals underwent cardiac catheterization, hemodynamic assessment, and Melody ViR implantation via a trans-femoral venous, trans-atrial septal approach, 1 week following surgery. Follow-up hemodynamic, angiographic, and echocardiographic data were recorded.
Results
Melody ViR implantation was technically successful in all but one animal. In this animal a 26 mm partial AR proved too large for secure anchoring of the Melody valve. In the remaining 9 animals, fluoroscopy showed the Melody devices securely positioned within the annuloplasty rings. Echocardiography revealed no perivalvular leak, and angiography revealed no left ventricular (LV) outflow tract obstruction, vigorous LV function, and no aortic valve insufficiency. The median procedure time was 55.5 (range 45–78) minutes.
Conclusions
This study demonstrates the feasibility of a purely percutaneous approach to MV replacement in patients with preexisting annuloplasty rings, regardless of ring type. This novel approach may be of particular benefit to patients with failed repair of ischemic MR, and in pediatric patients with complex structural heart disease.
Keywords: Mitral Valve, Mitral Valve Repair, Mitral Valve Replacement, Mitral Regurgitation, Minimally Invasive Surgery
Introduction
Mitral valve repair (MVR) has become the preferred surgical therapy for mitral regurgitation (MR) irrespective of the cause of valve dysfunction. Restoration of the Mitral annulus to its normal size and shape using an annuloplasty ring (AR) is the most widely applied and reliable MVR procedure. Currently, there are several different types of AR available, which can be loosely divided into the following 4 general categories: 1) rigid complete “flat” rings; 2) partial rings; 3) flexible rings; and 4) saddle-shaped rings. While no randomized, prospective data exist, valve repair is thought to result in superior valve function, reduced need for anticoagulation and improved survival when compared to valve replacement[1]. Improved durability over bioprosthetic heart valves has also been proposed as an advantage of repair[1]. However, recent studies have called into question the durability of mitral valve repair for functional, ischemic, and degenerative etiologies[2–6], and durable repair of congenital systemic atrioventricular (AV) valve dysfunction (mitral, tricuspid or common AV valve) is equally problematic[7, 8].
Repair of ischemic mitral regurgitation (IMR) with undersized annuloplasty has an extremely high rate of early recurrent MR with up to 30% of patients suffering return of hemodynamically important MR within 6 months of surgery[2, 9]. Recurrence of significant MR after repair for degenerative disease has also been documented to approach 4% per year in some reports[3–5, 9, 10]. Due to very complex pathologies, recurrence of regurgitation following valve repair for congenital anomalies is also common[8, 11], necessitating high-risk surgical valve replacement in children, often with a mechanical device in the supraannular position[12].
Over the last decade, the development of novel valve technologies has made percutaneous replacement of the aortic and pulmonary valves a clinical reality[13–16]. However, percutaneous replacement of the mitral valve (MV) is not currently possible due to inherent anatomic features of the MV that make fixation and perivalvular seal with currently available devices a challenge. Recent clinical reports and animals studies have demonstrated that the presence of a surgically placed annuloplasty ring and/or a bioprosthetic valve makes MV replacement with valved-stents feasible with current devices. This technique has been termed the valve-in-ring (VIR) or valve-in-valve (VIV) procedure and has been performed surgically, via transatrial[17] and transapical[18–20] approaches using the Sapien® Transcatheter Heart Valve (Edwards Lifesciences, Irvine, California). Given the growing awareness of the limitations of valve repair durability, VIR offers a potential remedial procedure for adult and pediatric patients who develop recurrent MR after MV repair and are poor candidates for re-repair or conventional surgical valve replacement.
In this study, for the first time, we report on the feasibility of performing VIR via a completely percutaneous approach using the Melody® transcatheter valve (Medtronic, Minneapolis, MN) in a sheep model of mitral valve repair using 4 distinct types of annuloplasty ring, which are commonly used in MVR procedures. A purely percutaneous approach to the VIR procedure reduces the invasiveness of the procedure, which may be advantageous for high-risk patients.
Material and Methods
Following approval by The University of Pennsylvania’s Animal Care and Use Committee, 10 male Dorsett hybrid sheep were subjected to study. All animals had surgical mitral valve ring-annuloplasty. One week following surgery, all animals were brought to the catheterization laboratory for the percutaneous, transvenous Melody Valve-in-Ring (VIR) procedure. Hemodynamic and echocardiographic data were recorded before and after VIR.
Surgical Mitral Annuloplasty
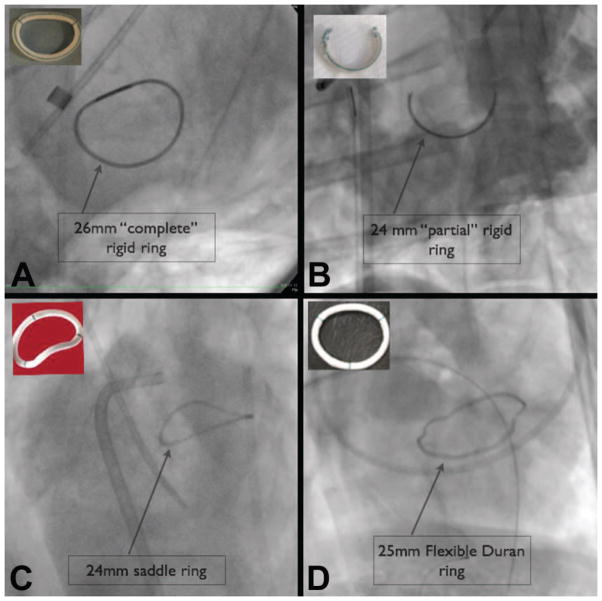
A left thoracotomy was performed, and one of 4 different annuloplasty rings was implanted in all animals using standard techniques (Figure 1):
Figure 1.
Figure 1 shows fluoroscopic images of the 4 types of annuloplasty rings used for the Melody VIR procedure, including: complete rigid rings (A); partial rings (B); flexible rings (C), and saddle rings (D). Photos of each ring are shown in the insets.
Complete rigid ring (Physio™, Edwards Lifesciences, Irvine, CA); 24mm [N=2]; 26mm [N=2]; 28mm ring [N=1]);
Partial rigid ring (modified Physio™); 24mm [N=2]; 26mm [N=1]
Saddle-shaped ring (Profile® 3D ring, Medtronic Inc., Minneapolis, MN); 24mm [N=1]
Flexible ring (Duran AnCore® ring, Medtronic Inc., Minneapolis, MN) 25mm [N=1]
Melody Valve-In Ring Procedure
One week following surgery, all animals were brought to the catheterization laboratory for the Melody VIR procedure. Vascular access was obtained via cut down for placement of a short (30cm) 22Fr vascular sheath (Cook Medical, Bloomington IN) into the right iliac vein. A 10Fr sheath was placed in the left femoral vein to facilitate intracardiac echocardiography (ICE), and arterial access was obtained in all animals via the carotid or femoral artery. Baseline fluoroscopy and angiography were performed to delineate the relevant anatomy and determine the annuloplasty ring dimensions. Under ICE guidance, the atrial septum was punctured and crossed using standard transeptal technique. A 12Fr sheath was advanced over a wire into the left atrium, and simultaneous left atrial (LA) and left ventricular (LV) pressures recorded. The atrial septal hole was enlarged via balloon dilation with a 15mm × 4cm Zmed2 balloon (NuMed Inc, Hopington, NY). ICE interrogation was used to document the size of the atrial septal defect (ASD). Next, an end hole catheter was advanced through the 12Fr sheath and into the LV, crossing through the center of the annuloplasty ring. Through the catheter, a pre-shaped, super-stiff 0.035” guidewire (Lunderquist® wire, Cook Medical, Bloomington, IN) was introduced and looped in the LV apex (Figure 2) creating a railway from the iliac vein to the LV via the ASD.
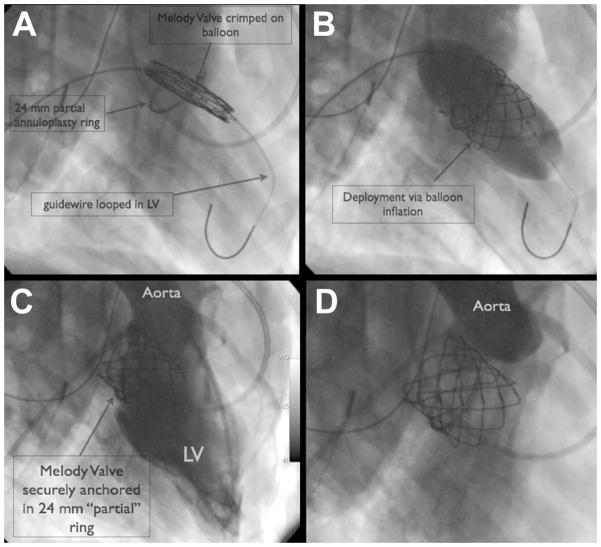
Figure 2.
Figure 2 shows the transvenous, transeptal approach to Melody Valve-in-Ring (VIR) delivery. The Melody device is crimped on the delivery balloon and advanced from the femoral vein, across the atrial septum and positioned centrally in the partial annuloplasty ring. Next, the Melody valve is deployed via balloon inflation. Follow-up angiography reveals no significant mitral regurgitation, no left ventricular outflow tract obstruction, and no aortic valve insufficiency. (LV = Left Ventricle)
In each animal, the Melody valves were crimped onto 22 mm diameter angioplasty balloons (22 mm × 4 cm BIB balloon Catheter, NuMed Inc. Hopington NY). The diameter of the balloon was chosen based on the known size limitations of the Melody Valve, which has a listed maximum functional diameter of 22mm. In one of the animals, a 28mm annuloplasty ring was placed. In this animal, the Melody device was intentionally over-dilated to 24–25 mm to ensure secure anchoring within the over-sized ring. To minimize the profile of the Melody valve, crimping was accomplished in a stepwise manner using Hegar dilators as previously described by our group[21]. The crimped Melody valve was advanced over the wire, through the 22Fr sheath and into the right atrium. Next, the device was advanced across the ASD into the LA and centered within the annuloplasty ring. Once in position, the device was deployed via standard balloon inflation technique (Figure 2). The guidewire was withdrawn from the left side of the heart across the ASD, and the small defect was closed with an ASD device (Helex®, Gore Medical or Amplatzer® Septal Occluder, AGA Medical). Post deployment angiography was performed to assess valve position and function. Follow up hemodynamics and echocardiography were recorded and compared to baseline values. Procedure time, defined as beginning after vascular access was established, and ending with ASD closure, was recorded for all animals. After a period of observation (6 hours), animals were euthanized as per protocol. Necroscopy was performed including gross inspection of the valve-in-ring complex.
Statistical Analysis
Hemodynamic measurements before and after VIR were summarized using standard descriptive statistics and reported as mean ± standard
Results
All animals [N=10] underwent successful surgical placement of an annuloplasty ring. There were no surgical or post-operative complications.
The only significant differences noted between the pre and post VIR hemodynamic measures were an increased cardiac output (p<0.01), and an increased systolic pulmonary artery pressure (p=0.04). Otherwise no significant differences were noted as shown in Table 1. Nine out of 10 (90%) VIR procedures resulted in secure Melody Valve implantation into the annuloplasty ring. The one failure occurred when implanting into the 26 mm partial annuloplasty ring. The Melody valve was too small to securely engage the partial ring and thus the device embolized during 2 separate deployment attempts. The median procedure time was 55.5 (range 45–78) minutes.
Table 1.
Hemodynamics
| Pre Valve-in- Ring (N=10) | Post Valve-in-Ring (N=9) | p- value | |
|---|---|---|---|
| Variable | |||
| Aortic systolic pressure(mmHg) | 98.7 ± 10.8 | 101.1 ± 11.7 | 0.66 |
| Aortic diastolic pressure(mmHg) | 57.7 ± 7.1 | 64 ± 16.2 | 0.32 |
| Aortic mean pressure | 72.5 ± 8.7 | 74.8 ± 12.7 | 0.67 |
| Heart rate (bpm) | 98.5 ± 17.1 | 104.4 ± 15.8 | 0.40 |
| LV systolic pressure (mmHg) | 99.2 ± 10.8 | 102.1 ± 11.2 | 0.53 |
| LV diastolic pressure(mmHg) | 12.3 ± 3.1 | 12.7 ± 3.8 | 0.44 |
| LA mean pressure (mmHg) | 16.9 ± 2.8 | 18.2 ± 3 | 0.34 |
| PCWp mean (mmHg) | 16.7 ± 2.4 | 17.7 ± 3.4 | 0.48 |
| PA systolic pressure (mmHg) | 32.5 ± 4.5 | 37.4 ± 5.4 | 0.04 |
| PA diastolic presure (mmHg) | 20.3 ± 4.0 | 21.3 ± 4.4 | 0.59 |
| PA mean pressure (mmHg) | 25.2 ± 3.5 | 28.1 ± 4 | 0.11 |
| RA pressure mean (mmHg) | 10.5 ± 3.7 | 12.2 ± 2.9 | 0.07 |
| Cardiac Output (L/min) | 4.3 ± 0.3 | 5.2 ± 0.7 | <0.001 |
| PVR (Wood Units) | 1.9 ± 0.9 | 1.9 ± 1.1 | 0.99 |
bpm = beats per minute; L/min = liters/min; LA = left atrium; LV = left ventricle; mmHg = millimeters of mercury; PA = pulmonary artery; PCWp = pulmonary capillary wedge pressure; PVR = Pulmonary Vascular Resistance; RV = right ventricle.
In the remaining animals, follow up angiography and echocardiography revealed excellent left ventricular systolic function. There was mitral regurgitation through a central coaptation defect in 6 animals: trivial in 2, mild in 3, and moderate-to-severe in 1. In this animal, the 28mm Physio™ ring was too large for secure anchoring of the Melody device when it was expanded to 22 mm. Therefore, this valve was intentionally over-expanded, resulting in malcoaptation of the valve leaflets and moderate-to-severe central MR.
There was no LV outflow tract obstruction (LVOTO), aortic insufficiency or perivalvular regurgitation in any animal (Table 1 and Figure 2).
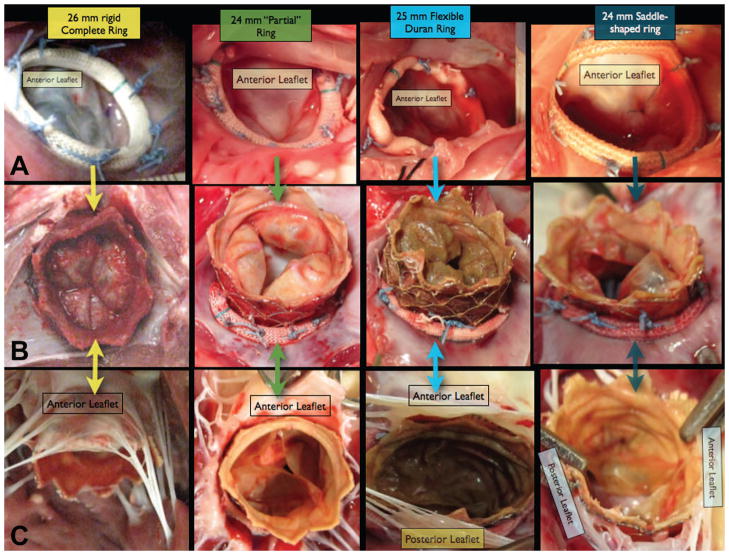
Following euthanasia, necropsy demonstrated that the Melody valves were anchored securely. There was a complete circumferential seal around the Melody valve stent, regardless of AR type (Figure 3).
Figure 3.
Row A shows the intraoperative images following surgical Mitral annuloplasty with 4 different types of annuloplasty ring. Row B shows necropsy pictures of the atrial side of the Melody Valve-in-Ring complex. Note that there is a complete circumferential seal formed between the Melody valve and the annular tissue, even in the absence of a complete ring (column 2). Row C shows the necropsy pictures of the ventricular side of the Melody Valve-in-ring complex. In all cases, the Mitral leaflet tissue “hugs” the outside of the Melody device. Although the Melody valve leaflets appear “knarled” and “rolled”, in most cases the valves functioned well, with only one having hemodynamically significant Mitral regurgitation.
Comment
In this report we describe the first transvenous, Valve-In-Ring implantation procedure, using the Melody® Valve in combination with 4 distinct classes of annuloplasty ring (AR): 1) Rigid complete ring; 2) Partial ring; 3) Flexible ring; and 4) Saddle-shaped ring. The Melody valves were securely implanted in all cases except for one (90%), with no perivalvular leakage noted, and not greater than trivial to mild central MR excluding the one animal in which we intentionally oversized the device. Importantly, there was no LV outflow tract obstruction, nor aortic valve insufficiency noted following VIR insertion. The success and relative ease of this procedure irrespective of AR type, highlights the potential for this approach in patients with ongoing MV dysfunction despite prior surgical repair.
Many promising percutaneous mitral valve (MV) repair technologies have been developed [5, 22]. However, one-stage percutaneous MV replacement remains elusive [23, 24]. Minimally invasive surgical (transapical or transatrial) MV replacement via valve-in-valve (VIV) and more recently valve-in-ring (VIR) procedures have been published [17, 20], which underscore the potential of repurposing “failing” surgical hardware (bioprosthetic valves and annuloplasty rings) as “landing zones” for subsequent minimally invasive valve replacement. Our experience supports and further refines this concept and demonstrates that a purely percutaneous approach to VIR implantation is feasible, in all types of annuloplasty devices, including, complete, partial, rigid, flexible, and saddle-shaped rings.
In patients with ischemic mitral regurgitation (IMR) the VIR procedure has the potential to be truly transformative. The optimal treatment for IMR remains controversial. The recent documentation of high recurrence rates of IMR after ring annuloplasty[5] has resulted in equipoise between the relative merits of repair versus replacement in this expanding cohort of patients. In fact there is an ongoing NIH funded clinical trial conducted within the Cardiothoracic Surgical Trials Network that has been developed to assess whether repair or replacement are best suited for patients with severe IMR. The availability of a fully percutaneous VIR procedure that is safe, fast, and effectively reframes the debate between repair and replacement in IMR. Annuloplasty could be performed in nearly all patients, maximizing the number who would experience the potential benefits of repair, while at the same time setting the stage for a quick, minimally-invasive remedial procedure–VIR - should symptomatic MR recur after surgery.
Limitations
This was a proof of principle study designed to test the feasibility of percutaneous VIR into four different mitral AR types. The Melody® valves used in this experiment will in all likelihood not be the optimal design for treating the majority of patients with failed mitral valve repair procedures since the majority of patients having such surgeries receive annuloplasty devices with nominal sizes of 28 mm or greater. We believe that the VIR procedure will be an important addition to the treatment of recurrent MR and believe that a product with a wide variety of sizes needs to be designed specifically for this indication.
Additionally, the devices were previously handled and thus not optimal for functionality testing. Furthermore, they are undersized relative to the normal adult mitral annulus (max functional diameter = 22mm), and are not intended for use in the systemic circulation, where the after load is generally much higher than in the pulmonary circulation. Despite this, the Melody valves were sufficient to demonstrate the feasibility of the transvenous VIR procedure in all types of annuloplasty rings, and the functioned well in the short term. This was an acute study, therefore we cannot comment on the durability of Melody devices in the Mitral position in the longer term.
Conclusions
This report demonstrates a purely percutaneous, transvenous approach to mitral VIR replacement using the Melody valve in 4 different types of annuloplasty ring. The relative ease of access to the mitral space from the venous side with standard catheter sizes and techniques is encouraging in terms of the feasibility of this approach in patients. All reports to date describing the VIR and VIV procedures, including this one, have employed valves that were developed for use in either the aortic or pulmonary positions; it is likely that devices designed specifically for mitral VIR and VIV procedures will further increase the ease of valve deliverability, durability and functionality in the mitral position, potentially resulting in widespread acceptance of this new treatment strategy.
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD (HL63954, HL73021 and 103723). R. Gorman and J. Gorman are supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX. The Melody valves used in this study were donated by Medtronic, Inc. (Minneapolis, MN). The authors had full control of the design of the study, methods used, outcome parameters and results, analysis of data and production of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 2.McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003;107:1609–1613. doi: 10.1161/01.CIR.0000058703.26715.9D. [DOI] [PubMed] [Google Scholar]
- 4.Flameng W, Meuris B, Herijgers P, Herregods MC. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg. 2008;135:274–282. doi: 10.1016/j.jtcvs.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Feldman T, Foster E, Glower DG, et al. Percutaneous Repair or Surgery for Mitral Regurgitation. N Engl J Med. 2011 doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 6.Hoashi T, Bove EL, Devaney EJ, Hirsch JC, Ohye RG. Mitral valve repair for congenital mitral valve stenosis in the pediatric population. Ann Thorac Surg. 2010;90:36–41. doi: 10.1016/j.athoracsur.2010.03.099. [DOI] [PubMed] [Google Scholar]
- 7.Mahle WT, Cohen MS, Spray TL, Rychik J. Atrioventricular valve regurgitation in patients with single ventricle: impact of the bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2001;72:831–835. doi: 10.1016/s0003-4975(01)02893-4. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Gaynor JW, Spray TL. Atrioventricular valve replacement in patients with a single ventricle. Ann Thorac Surg. 2001;72:182–186. doi: 10.1016/s0003-4975(01)02699-6. [DOI] [PubMed] [Google Scholar]
- 9.Anyanwu AC, Adams DH. Why do mitral valve repairs fail? J Am Soc Echocardiogr. 2009;22:1265–1268. doi: 10.1016/j.echo.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg. 2007;19:90–96. doi: 10.1053/j.semtcvs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Atz AM, Hawkins JA, Lu M, et al. Surgical management of complete atrioventricular septal defect: Associations with surgical technique, age, and trisomy 21. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selamet Tierney ES, Pigula FA, Berul CI, Lock JE, del Nido PJ, McElhinney DB. Mitral valve replacement in infants and children 5 years of age or younger: evolution in practice and outcome over three decades with a focus on supra-annular prosthesis implantation. J Thorac Cardiovasc Surg. 2008;136:954–961. 961 e951–953. doi: 10.1016/j.jtcvs.2007.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 14.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 15.McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 122:507–516. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the Core Valve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation. 2006;114:1616–1624. doi: 10.1161/CIRCULATIONAHA.106.639450. [DOI] [PubMed] [Google Scholar]
- 17.Kempfert J, Blumenstein J, Chu MW, et al. Minimally invasive off-pump valve-in-a-ring implantation: the atrial transcatheter approach for re-operative mitral valve replacement after failed repair. Eur J Cardiothorac Surg. 2009;35:965–969. doi: 10.1016/j.ejcts.2009.02.018. discussion 969. [DOI] [PubMed] [Google Scholar]
- 18.Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–1857. doi: 10.1161/CIRCULATIONAHA.109.924613. [DOI] [PubMed] [Google Scholar]
- 19.Walther T, Kempfert J, Borger MA, et al. Human minimally invasive off-pump valve-in-a-valve implantation. Ann Thorac Surg. 2008;85:1072–1073. doi: 10.1016/j.athoracsur.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Casselman F, Martens S, De Bruyne B, Degrieck I. Reducing operative mortality in valvular reoperations: The “valve in ring” procedure. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 21.Robb JD, Harris MA, Minakawa M, et al. Melody valve implantation into the branch pulmonary arteries for treatment of pulmonary insufficiency in an ovine model of right ventricular outflow tract dysfunction following tetralogy of Fallot repair. Circ Cardiovasc Interv. 2011;4:80–87. doi: 10.1161/CIRCINTERVENTIONS.110.959502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc Interv. 2011;4:1–13. doi: 10.1016/j.jcin.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Boudjemline Y, Pineau E, Borenstein N, Behr L, Bonhoeffer P. New insights in minimally invasive valve replacement: description of a cooperative approach for the off-pump replacement of mitral valves. Eur Heart J. 2005;26:2013–2017. doi: 10.1093/eurheartj/ehi307. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Tozzi P, Huber CH, Taub S, Gerelle G, von Segesser LK. Double-crowned valved stents for off-pump mitral valve replacement. Eur J Cardiothorac Surg. 2005;28:194–198. doi: 10.1016/j.ejcts.2004.12.068. discussion 198–199. [DOI] [PubMed] [Google Scholar]