Abstract
Thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) has recently been introduced as an alternative to 5-bromo-2-deoxyuridine (BrdU) for cell labeling and tracking. Incorporation of EdU into replicating DNA can be detected by azide-conjugated fluors (eg, Alexa-azide) through a Cu(i)-catalyzed click reaction between EdU's alkyne moiety and azide. While this cell labeling method has proven to be valuable for tracking transplanted stem cells in various tissues, we have found that some bone marrow cells could be stained by Alexa-azide in the absence of EdU label. In intact rat femoral bone marrow, ∼3% of nucleated cells were false-positively stained, and in isolated bone marrow cells, ∼13%. In contrast to true-positive stains, which localize in the nucleus, the false-positive stains were cytoplasmic. Furthermore, while true-positive staining requires Cu(i), false-positive staining does not. Reducing the click reaction time or reducing the Alexa-azide concentration failed to improve the distinction between true- and false-positive staining. Hematopoietic and mesenchymal stem cell markers CD34 and Stro-1 did not co-localize with the false-positively stained cells, and these cells' identity remains unknown.
Introduction
Thymidine analogs have been used extensively for analyzing DNA synthesis and for tracking cells after their transplantation into a host [1]. The importance of these thymidine analogs in biomedical research is best exemplified by the usage of 5-bromo-2-deoxyuridine (BrdU) in more than 20,000 peer-reviewed studies [1]. However, the histological detection of BrdU requires harsh conditions that affect cell morphology and protein antigenicity. In addition, the resulting histological images can be difficult to assess due to high background. As such, in 2008, Salic and Mitchison introduced a new thymidine analog, 5-ethynyl-2′-deoxyuridine (EdU), which can be easily detected without affecting cell morphology or protein antigenicity [2].
EdU is structurally similar to thymidine except that a terminal alkyne group replaces a methyl group at the 5 position of the pyrimidine ring. EdU can be incorporated into cellular DNA during the S phase and subsequently detected by an azide-conjugated fluor (commonly Alexa-azide). The azide–alkyne reaction, which is catalyzed by Cu(i), is one of the most popular within the click chemistry, and is alternatively called the Huisgen's reaction or cycloaddition [1,2].
While Salic and Mitchison's study focused on the detection of EdU in cultured cells, we extended EdU's usefulness to the tracking of exogenously transplanted cells and to the identification of endogenously proliferating cells in animals [3]. We have since used this technique in 18 published studies, including 2 recent ones that focused on the identification of label-retaining cells (LRC) in adipose tissue and in the urinary bladder, respectively [4,5]. Since the LRC technique is traditionally performed with BrdU for the identification of potential tissue-resident stem cells, our studies further extended EdU's utility to the localization of such cells.
In the above-mentioned LRC studies, rats were intraperitoneally injected with EdU, and at different time points their tissues were harvested for the detection of EdU-labeled cells by using a Click-iT kit containing red fluor Alexa594-azide (Invitrogen, Inc., Carlsbad, CA). Although adipose tissue and the urinary bladder were the respective focuses of these 2 studies, several other tissues were also harvested in an effort to maximize the contribution of the EdU-injected rats. Histological examination of these tissues expectedly found an inverse relationship between the number of EdU-positive cells and the length of time when the animal was sacrificed after EdU injection. However, in bone marrow, the EdU-positive cells were consistently more persistent than in other tissues. Thus, we decided to investigate the bone marrow of rats without EdU injection, and, indeed, we found many cells stained by Alexa594-azide. The evidence and additional data are presented herein.
Methods
Animals and EdU injection
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the University of California San Francisco. Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Newborn (within 1 h after birth) and 8-week-old (adult) rats were intraperitoneally injected with 160 mg/kg of EdU or phosphate-buffered saline (PBS). At different time points, various organs were harvested and processed for histological or cytometric analysis. Data presented in this article were obtained with penises of newborn rats at 24 h post-EdU injection and with the femoral bone marrows of newborn and adult rats at 24 h or 8 weeks post-EdU or post-PBS injection.
Histology
Rat femoral bone marrow and penis were fixed for 4 h with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in 30% sucrose. The fixed tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA) and stored at −80°C until use. Frozen tissues were cut at 6 μm, mounted to charged slides, and air-dried for 5 min. The tissue sections were washed in PBS followed by 30-min room-temperature incubation with 3% goat serum/PBS/0.3% triton X-100. The tissue sections were then incubated with a freshly made Click-iT reaction cocktail containing Alexa594-azide or Alexa488-azide (cat# C10339 and C10337, respectively; Invitrogen) for 30 min at room temperature. In certain experiments, as indicated in the Results section, certain components of the Click-iT reaction cocktail were omitted, or the incubation time was shortened, or the Alexa-azide concentration was reduced, or the Alexa-azide was replaced with either Alexa594-IgG or Alexa488-IgG (cat# A11012 and A11018, respectively; Invitrogen). Afterward, the tissues were stained for 5 min with 4′,6-diamidino-2-phenylindole (DAPI; for nuclear stain, Cat# D3571; Invitrogen). The stained tissues were examined with a Nikon Eclipse E600 fluorescence microscope and photographed with a Retiga 1300 Q-imaging camera using ACT-1 software (Nikon Instruments, Inc.). Individual images generated from the green, red, and blue channels were superimposed to generate the composite figures. Red- and blue-stained cells were counted in 5 random fields at 400×, and their ratio calculated as the percentage of positively stained bone marrow cells.
Isolation and flow cytometric analysis of bone marrow cells
Newborn rats were injected with EdU or PBS; 24 h later, their femoral bone marrows were flushed out with PBS, incubated in 0.75% collagenase at 37°C for 20 min, washed 3 times with PBS, and treated in a fixation and permeabilization solution (BD Biosciences, San Jose, CA) at room temperature for 10 min. Afterward, the individualized bone marrow cells were washed with 3% bovine serum albumin and incubated with anti-CD34 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-Stro-1 antibody (R&D Systems, Inc., Minneapolis, MN) in 50 μL buffer (1% FBS and 0.1% Na3N in PBS) for 30 min on ice. The cells were then incubated with Alexa594-IgG for 30 min on ice, followed by incubation in the Click-iT reaction cocktail containing Alexa488-azide for 30 min at room temperature without light. Thereafter, the cells were washed with PBS, resuspended in 2 mL of PBS, and analyzed in a fluorescence-activated cell sorter (FACSVantage SE System; BD Biosciences). The resulting data were further analyzed with FlowJo software (Tree Star, Inc., Ashland, OR) to determine the percentage of CD34, Stro-1, and EdU-positive cells among 1×105 bone marrow cells.
Isolation and staining of bone marrow cell nuclei
Adult rats were injected with EdU or PBS; 24 h later, their femoral bone marrows were flushed out with PBS, incubated in 0.75% collagenase at 37°C for 20 min, and washed 3 times with PBS. Half of each cell sample was resuspended in PBS and incubated on ice as normotonic control. The other half was resuspended in hypotonic solution (10 mM HEPES pH 7.9, 3 mM MgCI2, 10 mM KCI, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated for 5 min on ice. The bone marrow cell nuclei were collected by centrifugation at 500 rpm at 4°C for 5 min, washed twice with hypotonic solution, and resuspended in PBS. Both the normotonically and hypotonically treated cells were stained with the Click-iT reaction cocktail containing Alexa594-azide for 30 min at room temperature followed by incubation with DAPI for 5 min. The cells and nuclei were then examined with the Nikon Eclipse E600 fluorescence microscope and photographed with the Retiga 1300 Q-imaging camera using ACT-1 software. Individual images generated from the red and blue channels were superimposed to generate the composite figures. Red- and blue-stained cells and nuclei were counted in 5 random fields at 400×, and their ratio was calculated as the percentage of positively stained bone marrow cells.
Statistical analysis
Data were analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA) and expressed as mean±standard error of the mean for continuous variables. The continuous data were compared the groups using one-way analysis of variance. The Tukey–Kramer test was used for post-hoc comparisons. Statistical significance was set at p<0.05.
Results
Bone marrow is stained by Alexa-azide in the absence of EdU
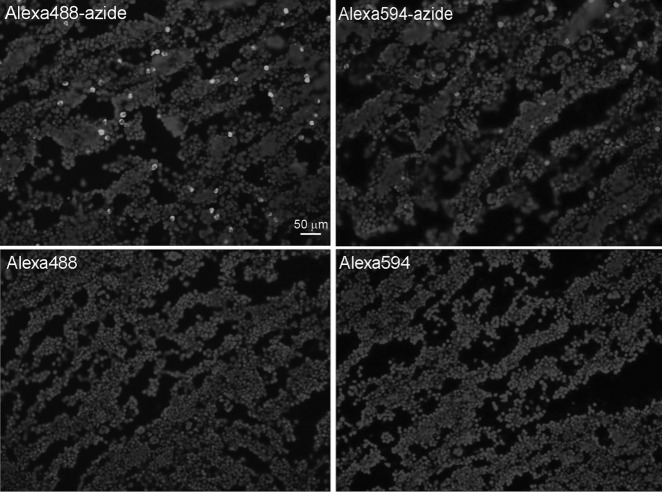
The femoral bone marrow of a normal adult rat without EdU injection was positively stained by Alexa488-azide or Alexa594-azide but not by the same fluors without azide (Fig. 1). For ease of data presentation and discussion, positive staining in the absence of EdU labeling will be called false-positive.
FIG. 1.
Bone marrow of an adult rat without 5-ethynyl-2′-deoxyuridine (EdU) injection was stained for 30 min with the Click-iT kit containing the indicated Alexa fluor, or green), followed by nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI).
By counting the red Alexa594-azide and the blue DAPI stains, we determined that 2.96%±0.78% (red versus blue) of nucleated bone marrow cells were false-positively stained by Alexa594-azide. On the other hand, in the femoral bone marrow of an adult rat that was injected with EdU 24 h earlier, 17.92%±3.20% of nucleated bone marrow cells were stained by Alexa594-azide.
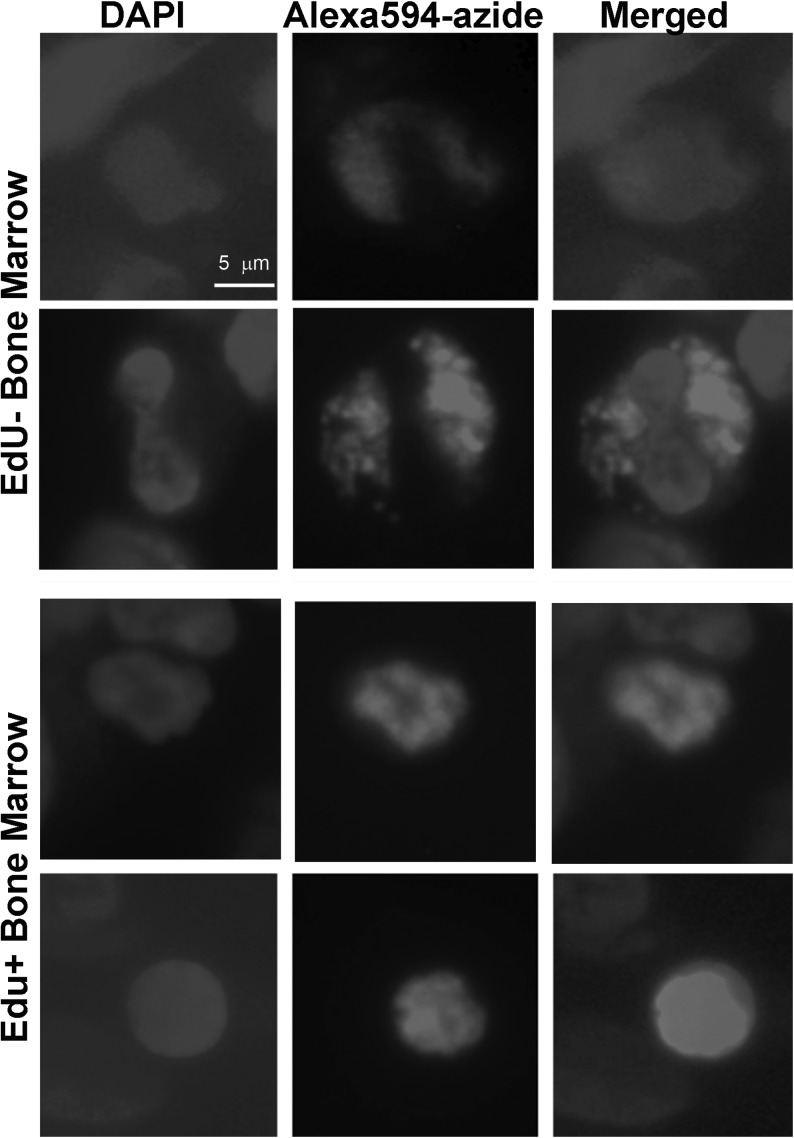
We have also found that the false-positive stains appeared to be cytoplasmic, whereas the true-positive stains, nuclear (Fig. 2). By counting the red cytoplasmic stains and the blue DAPI stains, we determined that 3.17%±1.01% of nucleated bone marrow cells in the EdU-injected rat were false-positively stained. This number was nearly the same as that (2.96%±0.78%) determined from the bone marrow of rat without EdU injection.
FIG. 2.
Bone marrows of adult rats injected 24 h earlier with phosphate-buffered saline (PBS) (labeled as EdU−), or EdU were stained for 30 min with the Click-iT kit containing Alexa594-azide.
The false-positive stains are cytoplasmic
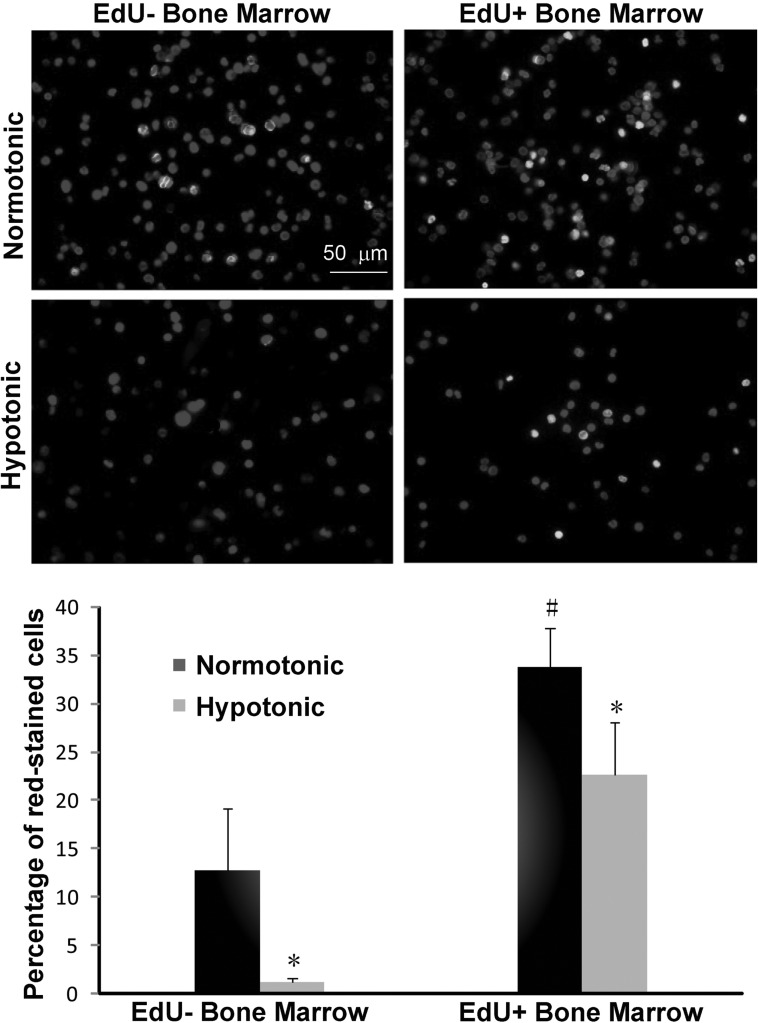
To verify that the false-positive stains were cytoplasmic, we isolated bone marrow cells from adult rats with and without EdU injection and lysed their cytoplasmic membrane in hypotonic solution before staining with Alexa594-azide. This hypotonic treatment resulted in the removal of nearly all false-positive stains in both the EdU-negative and EdU-positive bone marrow cell preparations (Fig. 3). Specifically, the percentage of positively stained cells in EdU+ bone marrow decreased from 33.80±4.10 to 22.70±3.50, and that in EdU− bone marrow from 12.85±6.30 to 1.30±0.32.
FIG. 3.
Isolated bone marrow cells of adult rats injected 24 h earlier with PBS (labeled as EdU−), or EdU were treated in normotonic or hypotonic solution and then stained for 30 min with the Click-iT kit containing Alexa594-azide. The percentages of stained cells are summarized in the bar chart. *Normotonic versus hypotonic, P<0.0001; #EdU− versus EdU+, P=0.0025.
The false-positive stains occur in the absence of Cu(i), reaction buffer, or additive
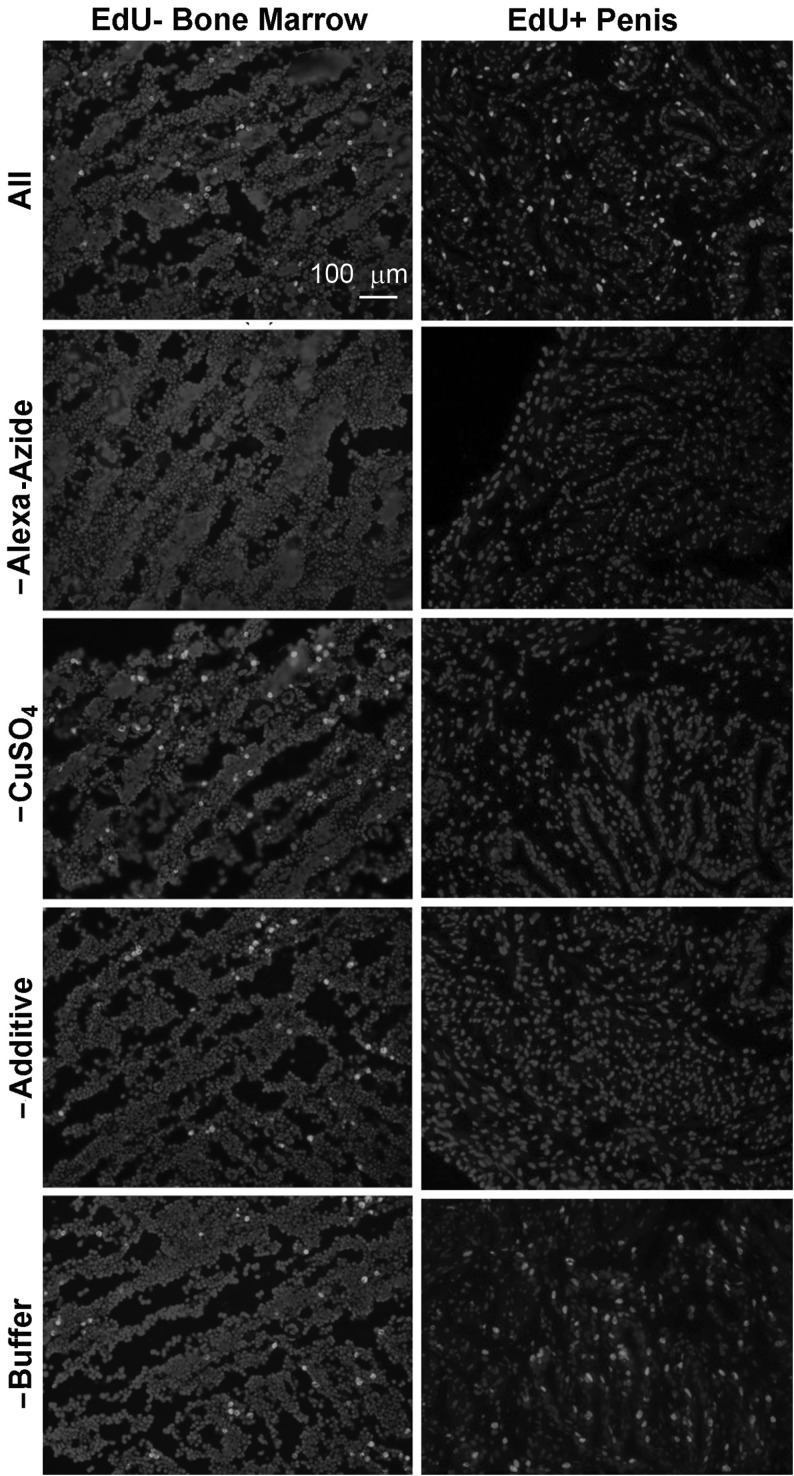
To investigate which component in the Click-iT kit was responsible for the false-positive stain in bone marrow, we conducted the click reaction by omitting 1 component at a time. As a positive control, penis of an EdU-injected rat was used for comparison. The results show that, for staining of EdU+ penis, each component was required except for the reaction buffer, which could be replaced with distilled water. On the other hand, the false-positive stains in the EdU− bone marrow occurred under every experimental condition except when Alexa-azide was omitted (Fig. 4).
FIG. 4.
Bone marrow of a rat without EdU injection and penis of a rat with EdU injection (harvested at 24 h post-injection) were stained for 30 min with a complete Click-iT kit containing Alexa594-azide (indicated as All) or with a partial Click-iT kit (indicated with the missing component), followed by nuclear staining with DAPI.
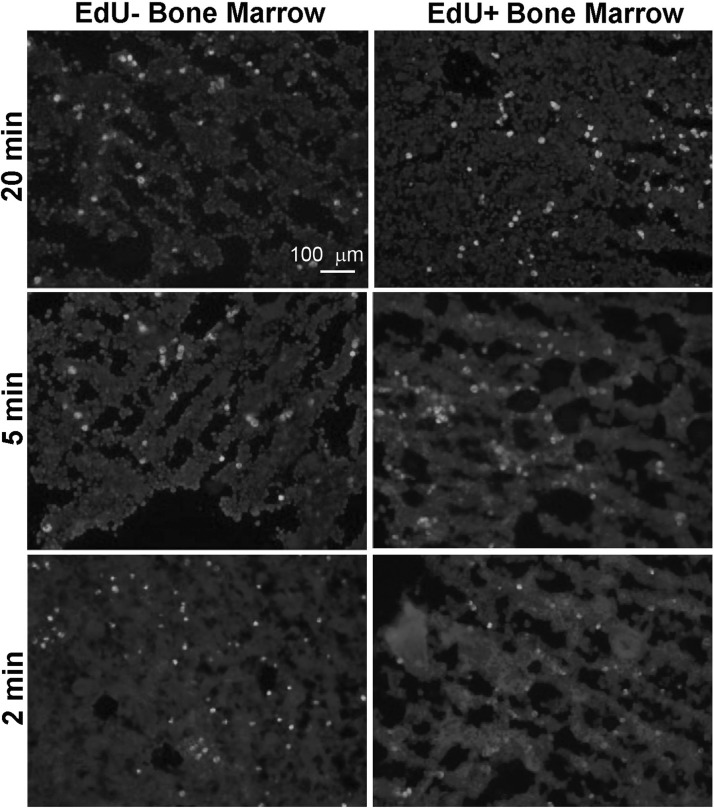
Reducing the reaction time does not reduce false positivity
We investigated whether reducing the reaction time could reduce the false-positive stain. The results show that reducing the reaction time affected the staining level in both the EdU+ and EdU− bone marrows and thus did not improve the staining specificity (Fig. 5).
FIG. 5.
Bone marrows of adult rats injected 24 h earlier with PBS (labeled as EdU−), or EdU were stained with the Click-iT kit containing Alexa594-azide for the indicated time.
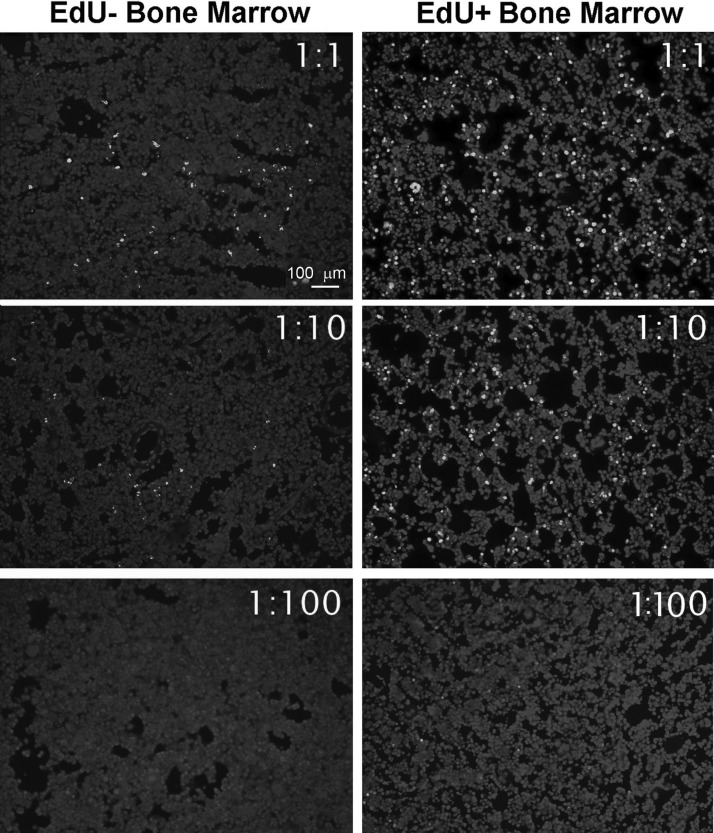
Reducing Alexa-azide concentration does not reduce false positivity
We also investigated whether lowering the Alexa-azide concentration could reduce the false-positive stain. The results show that reducing the Alexa-azide concentration affected the staining level in both the EdU+ and EdU− bone marrows, and thus did not improve the staining specificity (Fig. 6).
FIG. 6.
Bone marrows of adult rats injected 24 h earlier with PBS (labeled as EdU−), or EdU were stained for 30 min with the Click-iT kit containing serially diluted Alexa594-azide.
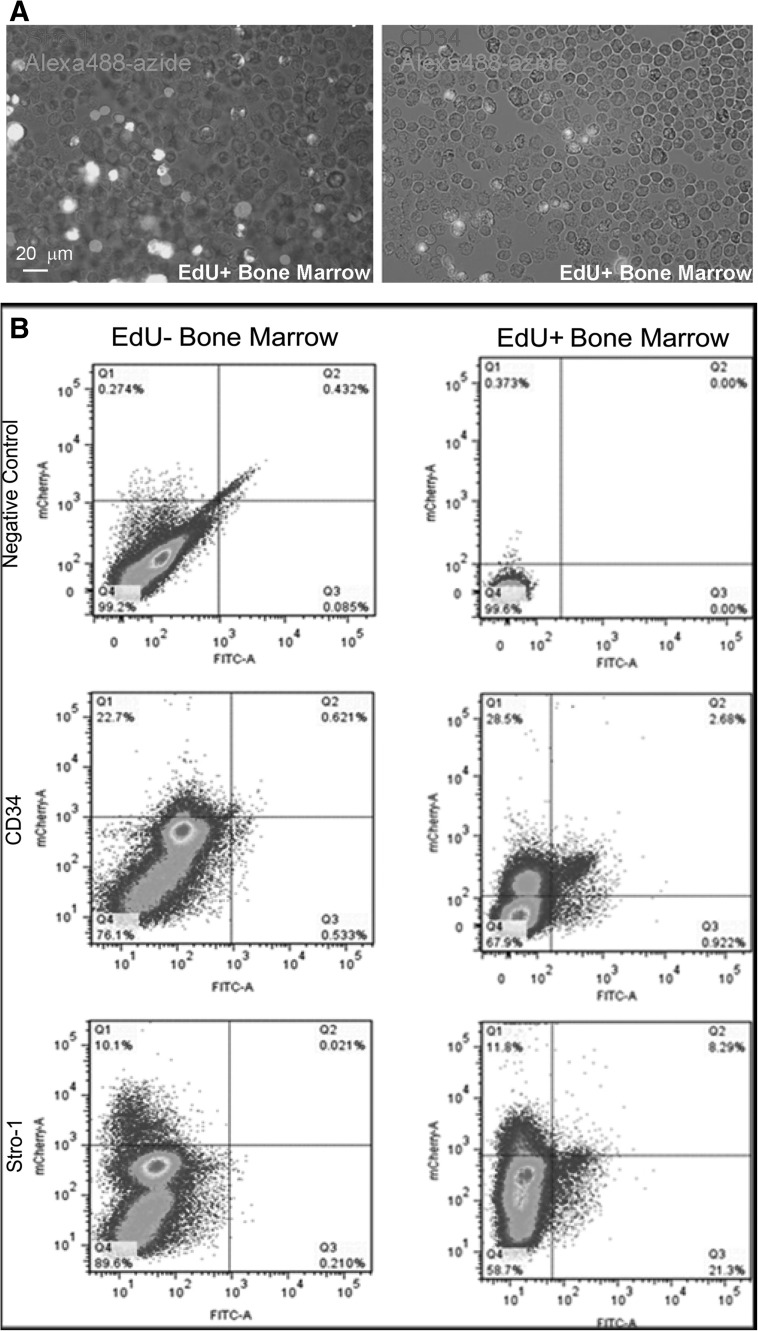
False-positive staining was not correlated with Stro-1 or CD34 expression
To investigate what cell types were false-positively stained, we prepared bone marrow cell suspensions and immunostained them with mesenchymal and hematopoietic stem cell markers Stro-1 and CD34, respectively. The results show that there was no correlation between marker expression and false-positive stain (Fig. 7).
FIG. 7.
Bone marrows of 1-day-old rats injected 24 h earlier with PBS (labeled as EdU−), or EdU were reacted with negative control, anti-CD34, or anti-Stro-1 antibody followed by reaction with Alexa594-conjugated secondary antibody and then stained with the Click-iT kit containing Alexa488-azide. Small portions of the EdU+ samples were examined microscopically (A). The remaining cell samples were analyzed by fluorescence-activated cell sorting (B).
Discussion
While most studies employed EdU for the analysis of DNA synthesis in cells, we and others [6,7] have used it for tracking transplanted cells or for visualizing proliferating cells in animal tissues. In these cell-tracking or cell-visualization experiments, we interpreted any positive stains as cells whose DNA has incorporated the EdU label. However, in our subsequent investigations we became suspicious of possible false-positive stains when we saw a less-than-expected decline of the number of stained cells in the bone marrow of rats that were injected with EdU several weeks earlier. Further examination of several tissues of rats without EdU injection confirmed our suspicion; that is, bone marrow, and bone marrow only, could be stained positive in the absence of EdU.
In all of our published studies involving EdU detection, we have used the red fluor Alexa594-azide, and this was also used in the above-mentioned false-positive stain experiment. In the present study, we repeated the experiment with the green fluor Alexa488-azide and found it to similarly stain the bone marrow of rats without EdU injection. Next, we wondered whether the false-positive stain was caused by nonspecific absorption of the Alexa fluor by bone marrow cells. We thus repeated the experiment with either Alexa594 or Alexa488 that was not conjugated to azide. The results indicated that azide was necessary for the false-positive stain to occur.
At magnifications 400×and higher, the Alexa-azide stains in EdU− bone marrows could be seen as wrapping around the cell nuclei, whereas those in EdU+ bone marrows mostly coinciding with the cell nuclei. By using this distinction, we determined that ∼3% of nucleated cells in the intact femoral bone marrow, with or without EdU labeling, were false-positively stained by Alexa-azide. As the false-positive stains appeared to be cytoplasmic, we proceeded to use a hypotonic treatment strategy to investigate this possibility. The results show that, by lysing the cytoplasmic membrane in hypotonic solution, the percentage of false-positively stained cells decreased from 12.85±6.30 (in normotonic solution) to 1.30±0.32 (in hypotonic solution). While this 10-fold decline largely confirmed the cytoplasmic localization of the false-positive stains, the staining rate of 12.85%±6.30% in the bone marrow cell preparation was much higher than that of 2.96%±0.78% in the bone marrow tissue section. This discrepancy can perhaps be explained by the difference in cell composition between the intact bone marrow and the isolated bone marrow cells. Specifically, the enzyme-based cell isolation procedure resulted in the preferential isolation of true bone marrow cells (eg, hematopoietic cells, as opposed to fibroblasts, and endothelial cells), thus enriching the Alexa-azide-reactive population. In other words, we believe that the false-positively stained cells reside in the bone marrow proper and not in the supportive or secondary structures.
Azide-mediated binding of fluor to EdU-labeled DNA requires Cu(i) as a catalyst [2]. Although no EdU was present in the false-positively stained bone marrow, we wondered whether Cu(i) was required for the false-positive stain to occur. Thus, we conducted the Click-iT reaction without CuSO4, and found that Alexa-azide could stain bone marrow cells in the absence of exogenously added Cu(i). Furthermore, we tried to block possible endogenous Cu(i) by treating the tissue section with ethylenediaminetetraacetic acid before reaction with the Click-iT kit, and the results were the same (data not shown). Finally, we decided to find out whether there was a condition in which false-positive staining would not occur. We conducted each experiment by omitting one component of the Click-iT kit each time, and the results showed that Alexa-azide could stain bone marrow cells in the absence of any Click-iT component. In contrast, each Click-iT component, except for the reaction buffer (replaceable with water), was necessary for Alexa-azide to stain EdU-labeled nonbone marrow tissues (in this case, penis).
The EdU detection protocol (mp10338; Invitrogen) stipulates a reaction time of 30 min. However, the click reaction is known for its speed; thus, we wondered whether reducing the reaction time could permit the differentiation between true- and false-positive stains. While we found that staining was completed in as short as 5 min, little or no difference was found between EdU+ and EdU− bone marrows. Next, we wondered whether reducing the Alexa-azide concentration could resolve the false-positive staining problem. We found that a 10-fold dilution of Alexa-azide worked nearly as well as undiluted Alexa-azide, but it did not allow us to distinguish between true- and false-positive stains.
From the above-described experiments, we concluded that certain bone marrow cells are inherently reactive to azide. But what are these cells? Knowing the complexity of bone marrow cell composition, it was difficult for us to decide what cell markers to test. Still, because we had investigated CD34 and Stro-1 expression in bone marrow recently [8], examining whether the azide-reactive cells express these 2 markers seemed a reasonable starting point. Flow cytometric analysis of bone marrow cells co-stained with Alexa488-azide and CD34 or Stro-1 indicated no co-localization. Thus, the identity of the false-positively stained cells requires further investigation.
The development of alkyne-based molecules as biological tools depends on the absence of naturally occurring alkyne-containing molecules in the target biological system. However, in the literature there is no clear answer to whether alkyne-containing molecules exist naturally. In our exhaustive literature search, we found only 1 article that discussed this issue, namely, the review article by Cavanagh et al., in which the authors stated, “terminal alkyne groups are rarely present in biological systems [1].” However, despite our exhaustive search, we are still unable to find any experimental evidence that alkyne-containing molecules exist naturally. Thus, based on our finding that the false-positive stain occurred with Alexa-azide but not with Alexa, we consider that this might be the first experimental evidence. However, how these cells could be stained rapidly by Alexa-azide without exogenously added Cu(i) is another puzzle that we hope can be solved someday by experts in the click chemistry.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK045370, DK64538, and DK069655).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cavanagh BL. Walker T. Norazit A. Meedeniya AC. Thymidine analogues for tracking DNA synthesis. Molecules. 2011;16:7980–7993. doi: 10.3390/molecules16097980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salic A. Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin G. Huang YC. Shindel AW. Banie L. Wang G. Lue TF. Lin CS. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11:864–873. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G. Xin Z. Zhang H. Banie L. Wang G. Qiu X. Ning H. Lue TF. Lin CS. Identification of active and quiescent adipose vascular stromal cells. Cytotherapy. 2011;14:240–246. doi: 10.3109/14653249.2011.627918. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H. Lin G. Qiu X. Ning H. Banie L. Lue TF. Lin CS. Label retaining and stem cell marker expression in the developing rat urinary bladder. Urology. 2011;79:746.e1–e6. doi: 10.1016/j.urology.2011.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehrehasa F. Meedeniya AC. Dwyer P. Abrahamsen G. Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Zeng C. Pan F. Jones LA. Lim MM. Griffin EA. Sheline YI. Mintun MA. Holtzman DM. Mach RH. Evaluation of 5-ethynyl-2'-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin G. Liu G. Banie L. Wang G. Ning H. Lue TF. Lin CS. Tissue distribution of mesenchymal stem cell marker Stro-1. Stem Cells Dev. 2011;20:1747–1752. doi: 10.1089/scd.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]