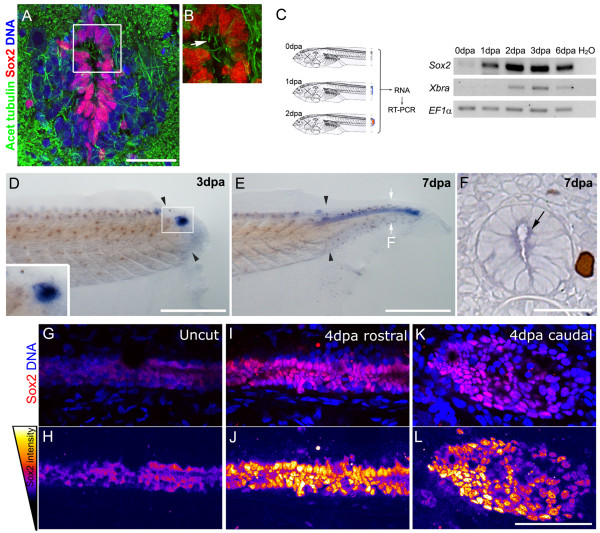
Figure 1.
Sox2 is upregulated after tail amputation. (A,B) Immunofluorescence of transverse sections of stage 49 tadpoles with anti-Sox2 (red) and anti-acetylated α-tubulin (green) antibodies; a 20 μm Z-projection was made to detect cilia (arrow). DNA was counterstained with TOTO3 (blue). (B) Magnification of the inset indicated in (A). (C) Reverse transcription polymerase chain reaction (RT-PCR) analysis of sox2 and Xenopus brachyury (Xbra) mRNA levels at the tip of the regenerating tail indicating that sox2 is upregulated after tail amputation. EF1α was used as loading control. (D-F)In situ hybridization using an antisense probe against sox2 during tail regeneration of stage 49 tadpoles at (D) 3, and (E,F) 7 days post amputation (dpa), sox2 was detected on the spinal cord and (D, inset) neural ampulla. (F) Transversal section of the regenerating tail at 7 dpa at the level indicated in (E), arrow indicates sox2 staining in the ependymal epithelium. (G-L) Sagittal optical section of whole-mount immunofluorescence with anti-Sox2 antibodies (red) before amputation (G,H) and at 4 dpa (I,J) rostral and (K,L) caudal regions. DNA was stained in blue. (H,J,L) Pseudocolored intensities of Sox2 channel from (G), (I) and (K), respectively as is described in the left scheme. Arrowheads: amputation plane. Scale bars: (A) 25 μm, (D,E) 400 μm, (F-L) 50 μm.