Abstract
Aim:
The aim was to assess the levels of HbA1c, C-reactive protein, and lipid profile in patients with type 2 diabetes mellitus by treating the pockets using collagen impregnated sustained release resorbable tetracycline fiber (periodontal plus AB fiber) following scaling and root planing (SRP).
Materials and Methods:
A total of 40 patients with type 2 diabetes mellitus were randomly distributed into two groups receiving either SRP and tetracycline fiber or SRP alone. Patients were evaluated clinically with gingival index, plaque index, probing depth, and relative attachment level, and bio-chemically with HbA1c, C Reactive Protein, and lipid profile at baseline, 1 month, and 3 months.
Results:
Significant reduction in all the clinical parameters was seen in the tetracycline group compared to the control group. Bio-chemical analysis also revealed similar results except for cholesterol and High density lipoprotein who did not show any significant reduction.
Conclusion:
Locally delivered tetracycline as a better treatment modality compared to SRP alone.
Keywords: Periodontitis, tetracycline hydrochloride, type2 diabetes
Introduction
Oral hygiene plays a critical role in whole-body health. Over the past decade, there has been an emerging interest in the interrelationship between systemic conditions and oral health.[1] As a front-line shield against systemic inflammation, one's oral status profoundly impacts the course and pathogenesis of a number of systemic diseases, such as diabetes mellitus, cardiovascular disease, bacterial pneumonia, and low birth weight.[2]
The American Society of Diabetes defined periodontal disease as the sixth complication of diabetes in 1997.[1,3] Diabetes mellitus is a complex disease with both metabolic and vascular components characterized by hyperglycemia due to defects in insulin secretion, insulin action or both.[4] It is a highly prevalent metabolic disorder with 150 million cases estimated world wide, constituting a global public health burden.[5]
Clinical and epidemiological studies have shown that patients with a long history of diabetes mellitus seem to have more periodontal breakdown than age-matched nondiabetic controls.[6]
The treatment implications in the management of periodontal disease as an integral component of diabetes care is in light of the current understanding of the pathogenesis of these two chronic conditions.[1] The mainstay of treatment for patients with periodontal disease involves mechanical methods—professional cleaning and mechanical débridement of the plaque or calculus, including both the supragingival and infragingival plaque.[7] But a successful treatment of periodontitis involves the ability to alter or eliminate the bacteria that cause the infection;[8] this led to the advent of antimicrobial in periodontal treatment. In order to obviate the negative effects attributed to systemically delivered antibiotics and to increase the concentration of chemotherapeutic agent in the GCF,[9] numerous attempts to locally deliver these agents have been made. Various mechanisms for local delivery of chemotherapeutic agent include subgingival irrigation, use of gels,[10] hollow fibers,[11] acrylic strips,[12] dialysis tubings,[13] and collagen preparations.
Among the various antibiotics, tetracycline-hydrochloride became popular in 1970s as a local delivery agent due to its broad spectrum antimicrobial activity, low toxicity, with additional properties like collagenase inhibition, antiinflammtory actions, inhibition of bone resorption, and promote attachment of fibroblasts.[14]
There is clear evidence that periodontal disease worsens glycemic control in diabetics[15] as well as inflammatory markers like CRP[16] and lipid metabolism.[17] However, further research is warranted to elucidate the effect of periodontal treatment on their control. Hence, the following study is planned to assess the levels of C-reactive protein, lipid profile, and HbA1c in patients with type 2 diabetes mellitus by treating the pockets using collagen impregnated sustained release resorbable tetracycline fiber (periodontal plus AB fiber) following scaling and root planing.
Materials and Methods
The study was conducted on patients visiting outpatient Department of Periodontology and Oral Implantology, I.T.S. Dental College and Hospital.
The study model comprised two groups with controlled or moderately controlled type 2 diabetes mellitus, with 20 patients in each group, age ranging from 35 to 70 years. Patients with chronic periodontitis with at least three sites with a probing depth of ≥4 mm and ≤7 mm were included in the study. Patients undergoing regular antiinflammatory medication, taken systemic antibiotics within last 3 months prior to enrollment, who have received periodontal therapy within last 6 months, tobacco users, major medical complications, less than eight natural teeth in oral cavity, pregnant and lactating women, and teeth with poor prognosis were excluded from the study.
Group 1 – 20 patients treated with scaling and root planing and placement of the tetracycline fiber at selected sites.
Group 2 – 20 patients treated with scaling and root planing alone.
The subjects had received detailed information regarding their condition and treatment plan. Detailed oral hygiene instructions were provided and written informed consent was taken from the patient.
Clinical parameters
Gingival index – Loe H and Silness J[18].
Plaque index – Turesky - Gilmore and Glickman modification of Quigley Hein Plaque index[19].
Pocket probing depth was recorded to nearest millimeter using UNC 15 probe. It was measured at four places around each tooth namely, mesiofacial, buccal, distofacial, and lingual [Figure 1].
Relative attachment levels in millimeter from reference point to the base of pocket using customized occlusal acrylic stents and UNC 15 probe[20] [Figure 2].
Figure 1.
Probing depth
Figure 2.
Relative attachment level
Treatment regimen
All subjects underwent periodontal examination by a single examiner.
Group 1: Subjects received standard periodontal therapy: scaling and root planing with ultrasonic scaler and Gracey's curettes. Scaling and root planing was followed by polishing using a low abrasive paste and oral hygiene instructions were given which was followed by local application of tetracycline fibers at the involved site [Figure 3]. This was followed by placement of a periodontal pack (*COE pack) for a period of 10 days. Patients were advised to follow oral hygiene instructions.
Figure 3.
Fiber placement
Group 2: Subjects received standard periodontal therapy: scaling and root planing with ultrasonic scaler and Gracey's curettes. Scaling and root planing was followed by polishing using a low abrasive paste and oral hygiene instructions were given.
Diabetes Mellitus-Related Variables
Three milliliter blood was collected and analyzed for glycosylated hemoglobin (HbA1c), C- reactive protein, and lipid profile at base line, 1 and 3 months except HbA1c which was analyzed at baseline and 3 months following scaling and root planing. All the tests were analyzed in a biochemical analyzer by Bayer. The methodology of storage, transport, and analysis is given in table 2. The HbA1c level was assessed to know the glycemic control of the patients with diabetes. The patients with HbA1c less than 8% were considered. CRP is an acute phase reactant used as a marker of systemic inflammation and as cardiovascular risk marker. Assessment of the lipid profile consisting of LDL, HDL, triglycerides, and total cholesterol was also done. The blood examination was done in one pathology lab to avoid variability in results.
Table 2.
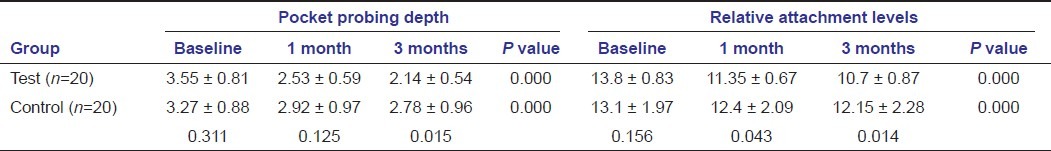
Mean ± SD of the pocket probing depth and CAL at different intervals
Statistical analysis
Data analysis was done using the SPSS software package. Intergroup comparison was done by using the Student t-test, whereas for intragroup comparison ANOVA, a paired t-test were used.
Results
Observed power at each point of time was 1, revealing the adequacy of sample size. All the subjects completed the study. The baseline readings at all point of time were not significant showing equality between the two groups.
All clinical parameters show significant reduction in the tetracycline group compared to the control group at 1 and 3 months except the probing depth [Tables 1 and 2]. Highly significant intergroup and intragroup reduction is seen in gingival scores revealing that tetracycline as an effective treatment modality in reducing gingivitis [Table 1]. Significant reduction in plaque scores were seen between the groups at 1 month and 3 months, with significant reduction in both the groups at baseline to 1 month and 3 months [Table 1]. Highly significant intragroup reduction is seen in probing depth at all point of time except 1--3 months in the control group; there is a significant reduction in the test group at 3 months time compared to the control group [Table 2]. On intragroup comparison, the relative attachment level was also reduced significantly at all point of time except 1--3 months in the control group, there is a significant reduction in the test group at 1 month and 3 months compared to the control group [Table 2].
Table 1.
Mean ± SD of the gingival and plaque index at different intervals
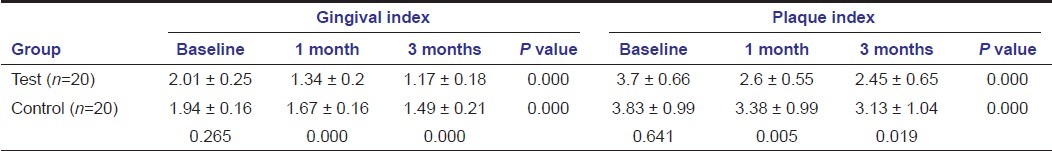
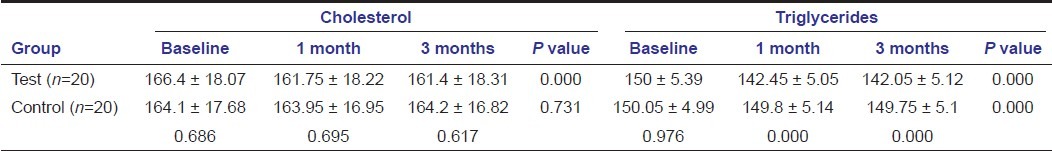
Also, on comparing bio-chemical parameters tetracycline showed better results compared to SRP alone as seen with HbA1c, CRP, and lipid profile except for total cholesterol and HDL at any point of time [Tables 3–5]. HbA1c levels showed significant change in both the groups when compared intra- and intergroup [Table 3]. Comparing C-reactive protein within the groups showed significant reduction from baseline to 1 month in both the groups, with significant reduction in the test group compared to the control group at 1 month and 3 months [Table 3]. Cholesterol levels showed significant reduction in the test group at all point of time, but not significant when compared to the control group [Table 4]. Triglycerides levels demonstrated significant reduction in the test group at all point of time and compared to the control group [Table 4]. Low-density lipoprotein levels showed significant reduction in the test group at all point of time and compared to the control group [Table 5]. High-density lipoprotein levels showed a significant change in the test group at all point of time, but not significant when compared to the control group [Table 5].
Table 3.
Mean ± SD of the HbA1c and CRP at different intervals
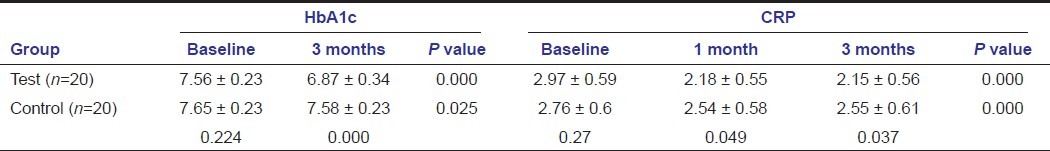
Table 5.
Mean ± SD of the LDL and HDL at different intervals
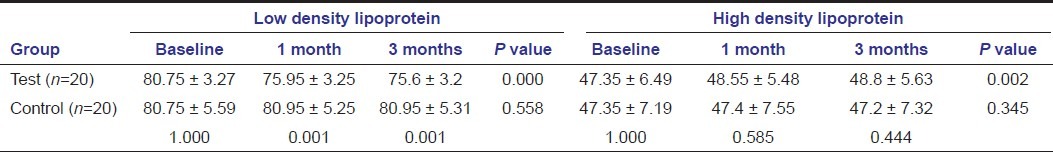
Table 4.
Mean ± SD of the CH and TG at different intervals
Discussion
SRP alone is an effective measure in reducing clinical parameters in diabetic patients as reported by Kiran et al.,[4] Smith et al.,[21] and Kardesler et al.[22] However, scaling and root planing become progressively less effective as the depth of the pocket increases to ≥5 mm as seen by Goodson et al.[23] Due to various disadvantages of systemic administration of drugs such as toxicity, adverse drug reaction, bacterial resistance, and low levels in gingival crevicular fluid, the focus is shifting toward local application of drugs. Magnusson et al. found that the periodontal pocket concentration of tetracycline to be several times higher than with systemic therapy.[24]
In the present study, a comparative evaluation had been done in type 2 diabetic patients with tetracycline fibers as an adjunct to scaling and root planing and scaling and root planing alone which is followed by analysis of biochemical markers.
The gingival score follow a decrease with time in both control and test group. This decrease was very highly significant in the test group than that in the control groups. The gingival score follow a decrease with time in both control and test groups, more so in the test group than that in the control group. These results were in agreement with those reported by Kiran et al.[4] and Cruz et al.[25] The reason could be the tetracycline antiinflammatory property as dictated by Seymour et al.[14] Plaque scores also showed significant reduction in both test and control groups from baseline with more significant reduction in the tetracycline group compared to the control group, as supported by Kiran et al.[4] Both the groups showed significant reduction in probing depth from baseline with more reduction with tetracycline compared to control at 3 months time. Similar results were reported by Rodrigues et al.,[26] Navarro-Sanchez et al., and[27] Cruz et al.[25] Significant attachment gain was seen in both the groups with more reported with tetracycline than that in the control group, as correlated with the work of Lima et al.,[28] Kiran et al.,[4] Navarro-Sanchez et al.,[27] and Cruz et al.[25]
Clinical findings were well supported by biochemical findings including C-reactive protein, lipid profile, and HbA1c. HbA1c showed highly significant mean decrease in the tetracycline group, with insignificant reduction seen in the control group at 3 months. This is similar to the findings of Faria-Almeida et al.,[15] Rodrigues et al.,[26] Navarro-Sanchez et al.,[27] Iwamoto et al.,[29] Al- Zahrani et al[30] with the use of systemic doxycycline, Kardesler et al.[22] These findings were inconsistent with results reported by Rodrigues et al.,[26] which could be because of different form of antimicrobials used and also there baseline readings were not similar in both the groups.
The C-reactive protein also showed a more downward trend with tetracycline compared to the control group. This finding being already supported by Auito et al.[16]
Triglyceride showed significant reduction with time with no improvements seen in controls. Also, it is significant when assessing between the groups. A significant improvement in LDL levels was seen in tetracycline groups, with a significant intergroup improvement at 1 and 3 months. Although some improvement is seen in tetracycline in total cholesterol and HDL levels, no improvements were seen in the control group. Intergroup comparisons were also insignificant. These results are in accordance with Cutler et al.[31] and Kiran et al.[4]
The findings from this study help to deduce that locally delivered tetracycline fiber is an effective treatment modality when used in adjunct with SRP in patients with type 2 diabetics.
Conclusion
Controlling periodontal infection plays an important part in the overall management of type 2 diabetes. Effective periodontal treatment resulted in lower clinical and biochemical parameters, confirming the existing interrelationship between diabetes mellitus and periodontitis. Therefore, periodontal treatment can be included as a diabetes preventive measure. Our study provided some evidence to demonstrate the effectiveness of locally delivered antimicrobials especially; tetracycline in periodontal pocket along with SRP in type 2 diabetics. Henceforth, prevention and control of periodontal disease along with use of antimicrobials must be considered as an integral part of diabetes control.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tan WC, Tay FB, Lim LP. Diabetes as a risk factor for periodontal disease: Current status and future considerations. Ann Acad Med Singapore. 2006;35:571–81. [PubMed] [Google Scholar]
- 2.Li X, Koltveit KM, Tronstand L, Olsen I. Systemic disease caused by oral infection. Clin Microbiol Rev. 2000;13:547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 4.Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Pontes-Andersen CC, Flyvberg A, Buschard K, Holmstrup P. Relationship between periodontitis and diabetes: Lessons from rodent studies. J Periodontol. 2007;78:1264–75. doi: 10.1902/jop.2007.060491. [DOI] [PubMed] [Google Scholar]
- 6.Llambes F, Silvestre FJ, Hernandez-Mijares A, Guiha R, Caffesse R. The effect of non-surgical periodontal treatment with or without doxycycline of type 1 diabetic patients. J Clin Periodontol. 2005;32:915–20. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 7.Herring ME, Shah SK. Periodontal disease and control of diabetes mellitus. J Am Osteopath Assoc. 2006;106:416–21. [PubMed] [Google Scholar]
- 8.Nassar H, Kantarci A, Van dyke TE. Diabetic periodontitis: A model for activated Innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007;43:233–44. doi: 10.1111/j.1600-0757.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malizia T, Tejada MR, Ghelardi E, Senesi S, Gabriele M, Giuca MR, et al. Periodontal tissue disposition of azithromycin. J Periodontol. 1997;68:1206–9. doi: 10.1902/jop.1997.68.12.1206. [DOI] [PubMed] [Google Scholar]
- 10.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 11.Yeung FI, Newman HN, Addy M. Subgingival metronidazole in acrylic resin vs chlorhexidine in control of chronic periodontitis. J Periodontol. 1983;54:651–6. doi: 10.1902/jop.1983.54.11.651. [DOI] [PubMed] [Google Scholar]
- 12.Greenstein G. Effects of sub-gingival irrigation on periodontal status. J Periodontol. 1987;58:827–36. doi: 10.1902/jop.1987.58.12.827. [DOI] [PubMed] [Google Scholar]
- 13.Pistorius A, Willershausen B, Steinmeier EM, Kreislert M. Efficacy of subgingival irrigation using herbal extracts on gingival inflammation. J Periodontol. 2003;74:616–22. doi: 10.1902/jop.2003.74.5.616. [DOI] [PubMed] [Google Scholar]
- 14.Seymour RA, Heasmann PA. Tetracyclines in the management of periodontal diseases. J Clin Periodontol. 1995;22:22–35. doi: 10.1111/j.1600-051x.1995.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 15.Faria-Almeida R, Navarro A, Bascones A. Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis. J Periodontol. 2006;77:591–8. doi: 10.1902/jop.2006.050084. [DOI] [PubMed] [Google Scholar]
- 16.D’aiuto F, Nibali L, Parker M, Suvan J, Tonetti MS. Short term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–73. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 17.Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: Recent concepts involving serum lipids. J Periodontol. 2000;71:1375–84. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 18.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 19.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of vitamin C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 20.Clark DC, Quee TC, Bergeron MJ, Chan EC, Lautar-Lemay C, Gruchy KD. Reliability of attachment level measurement using the CEJ and a plastic stent. J Periodontal. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 21.Smith GT, Greenbaum CJ, Johnson BD, Persson GR. Short-term responses to periodontal therapy in insulin-dependent diabetic patients. J Periodontol. 1996;67:794–802. doi: 10.1902/jop.1996.67.8.794. [DOI] [PubMed] [Google Scholar]
- 22.Kardeşler L, Buduneli N, Çetinkalp S, Kinaneadipokines DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol. 2010;81:24–33. doi: 10.1902/jop.2009.090267. [DOI] [PubMed] [Google Scholar]
- 23.Goodson JM. Pharmacokinetic principles controlling efficacy of oral therapy. J Dent Res. 1989;68:1625–32. [Google Scholar]
- 24.Magnusson I, Lindhe J, Yonayama T. Recolonization of subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11:193–207. doi: 10.1111/j.1600-051x.1984.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 25.Cruz GA, Toledo SD, Sallum EA, Sallum AW, Ambrosano GM, Sardi JD, et al. Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol. 2008;79:1150–7. doi: 10.1902/jop.2008.070503. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues DC, Taba M, Jr, Novaes AB, Jr, Souza LS, Grisi FM. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74:1361–7. doi: 10.1902/jop.2003.74.9.1361. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez A. Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol. 2007;34:835–43. doi: 10.1111/j.1600-051X.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 28.Lima AF, Cury CC, Palioto DB, Duro AM, Silva RC, Wolff LF. Therapy with adjunctive doxycycline local delivery with type 2 diabetes mellitus and periodontitis. J Clin Periodontol. 2004;31:648–53. doi: 10.1111/j.0303-6979.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto Y, Nishimura F, Nakagawa M, Sugimoto H, Shikata K, Makino H, et al. The effect of antimicrobial periodontal treatment on circulating TNF-α and glycated haemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72:774–8. doi: 10.1902/jop.2001.72.6.774. [DOI] [PubMed] [Google Scholar]
- 30.Al-Zahrani MS, Bamshmous SO, Alhassani AA, Al-Sherbini MM. Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol. 2009;80:1568–73. doi: 10.1902/jop.2009.090206. [DOI] [PubMed] [Google Scholar]
- 31.Cutler CW, Manchen RL, Jotwani R, Lacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipedimia. J Periodontol. 1999;70:1313–21. doi: 10.1902/jop.1999.70.11.1313. [DOI] [PubMed] [Google Scholar]


