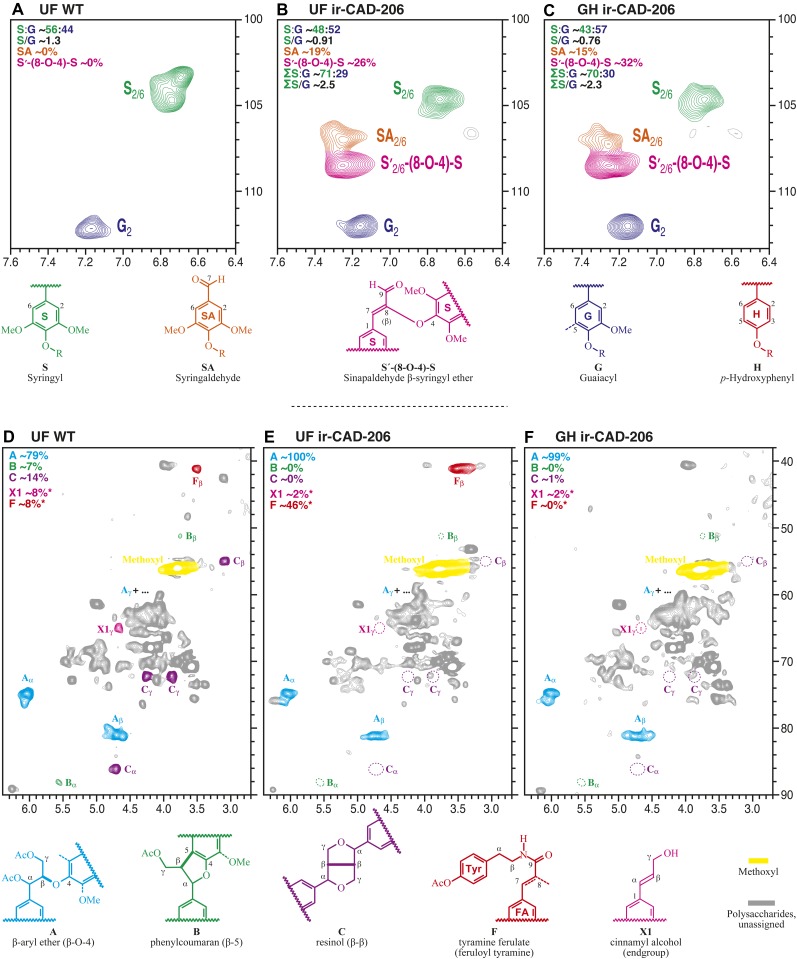
Figure 6.
A to C, Partial two-dimensional HSQC NMR spectra of UF wild-type (WT; A), UF ir-CAD (B), and GH ir-CAD (C) acetylated whole lignin released after cellulase treatment. ir-CAD plants grown under UF conditions incorporated lower amounts of hydroxycinnamyl alcohol moieties, namely monomers resulting in S (green) and G (blue) units into their lignins, than did the UF wild-type plants. ir-CAD plants accumulated similar levels of alcohol units both under GH and UF conditions. Characteristic signals (as indicated by coloring of the contours and the partial structures) reflect the cross-peaks of end group hydroxybenzaldehyde (primarily syringaldehyde) and incorporated sinapaldehyde moieties, which are signatures of ir-CAD lignin. D to F, Two-dimensional HSQC NMR spectra of acetylated lignin obtained from UF wild-type (D), UF ir-CAD (E), and GH ir-CAD (F) stems. Monolignol units are combined to produce different interunit linkages as well as characteristic end units, as depicted in wild-type lignin. The NMR signals reflect the frequency of different cross-linkage types of the monolignol units. Lignin isolated from ir-CAD stems (E and F) is largely devoid of all the typical minor units (phenylcoumaran B, resinol C, and cinnamyl alcohol end group X1) that are part of wild-type lignin but retain normal β-ether unit A as the major interunit structure, reflecting a different lignification pathway in which lignification is started via the hydroxybenzaldehydes (and perhaps hydroxycinnamaldehydes) instead of the usual monolignol dehydrodimerization. UF ir-CAD lignin (E) accumulated higher amounts of tyramine ferulate moieties, indicating increased stress in these plants.