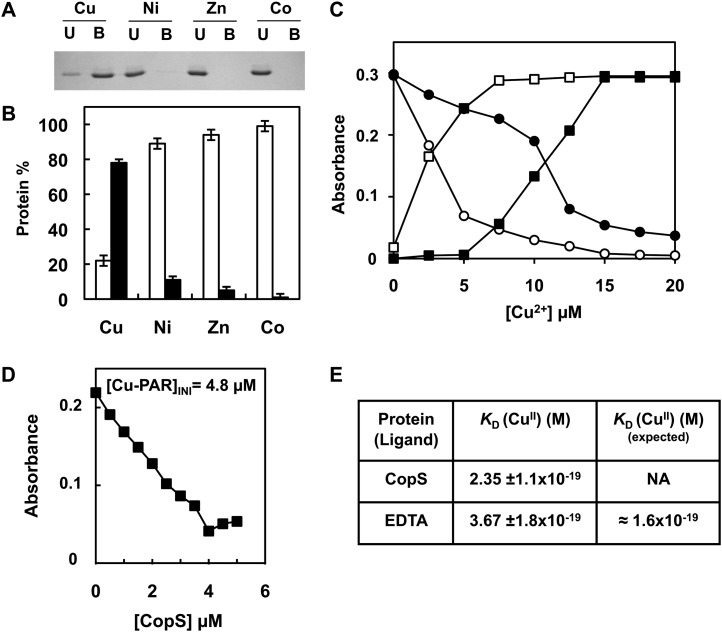
Figure 4.
CopS periplasmic domain binds copper. A, Analysis of CopS(38-183) protein interaction with metals. His-Bind resin columns were loaded with 0.5 mm CuSO4, NiSO4, ZnSO4, and CoCl2. About 10 μg of purified CopS(38-183) protein was applied to the columns. Unbound (U lanes) and bound (B lanes) fractions were analyzed by 15% SDS-PAGE and Coomassie Blue staining. B, Quantification of CopS in bound and unbound fractions. Coomassie-stained gel was scanned and the intensity of the bands was quantified using ImageJ program; the graph represents the average of two experiments. Unbound fraction (white), bound fraction (black). C, Titration of PAR, which absorbs at 410 nm (circles), to its copper form absorbing at 500 nm (squares), in the absence (white symbols) and presence (black symbols) of 10 μm CopS. D, Determination of the Cu2+ dissociation constant, KD, of CopS by titration into a solution of 10 μm PAR. The graph shows the decrease at 500 nm relative to CopS additions for a [Cu-PAR]TOTAL of 0.9 μm. E, Apparent KD CopS and EDTA at pH 7.5 derived from competition titration using Cu2+-PAR. Dissociation constant, KD, was estimated as described in “Materials and Methods” from four independent experiments like the one shown in D. NA, Not available.