Abstract
Background and Objectives:
Alpha-2 agonists are mixed with local anaesthetic agents to extend the duration of spinal, extradural and peripheral nerve blocks. We compared clonidine and dexmedetomidine as an adjuvant to local anaesthetic agent in supraclavicular brachial plexus block with respect to onset and duration of sensory and motor block and duration of analgesia.
Methods:
Sixty ASA I and II patients scheduled for elective upper limb surgeries under supraclavicular brachial plexus block were divided into two equal groups in a randomized, double-blinded fashion. Group C received clonidine 1 μg/kg and Group D received dexmedetomidine 1 μg/kg added to bupivacaine 0.25% (35 cc). Onset and recovery time of sensory and motor block, duration of analgesia and quality of block were studied in both the groups.
Results:
Duration of sensory block and motor block was 227.00±48.36 and 292.67±59.13 min, respectively, in group C, while it was 413.97±87.13 and 472.24±90.06 min, respectively, in group D. There was no statistically significant difference in onset of sensory and motor block between the two groups. The duration of analgesia (time to requirement of rescue analgesia) in group D was 456±97 min, while in group C, it was 289±62 min. Statistically, this difference was significant (P=0.001). The number of patients achieving grade IV quality (excellent) of block was higher in group D (80%) as compared with group C (40%) (P<0.05).
Conclusion:
Dexmedetomidine when added to local anaesthetic in supraclavicular brachial plexus block enhanced the duration of sensory and motor block and also the duration of analgesia. The time for rescue analgesia was prolonged in patients receiving dexmedetomidine. It also enhanced the quality of block as compared with clonidine.
Keywords: Clonidine, dexmedetomidine, supraclavicular block
INTRODUCTION
Upper limb surgeries are mostly performed under peripheral blocks such as the brachial plexus block. Peripheral nerve blocks not only provide intraoperative anaesthesia but also extend analgesia in the post-operative period without any systemic side-effects.[1]
There has always been a search for adjuvants to the regional nerve block with drugs that prolong the duration of analgesia but with lesser adverse effects. The search for the ideal additive continues, and led us to try the novel α2 adrenergic agent, dexmedetomidine.
Alpha-2 adrenergic receptor agonists have been the focus of interest for their sedative, analgesic, perioperative sympatholytic and cardiovascular stabilizing effects with reduced anaesthetic requirements. Furthermore, various methods of administration, such as epidural, intrathecal and peripheral injections, have been tried either alone or in combination with another drug to prolong and intensify the anaesthesia.[2–4]
Dexmedetomidine, a potent α2 adrenoceptor agonist, is approximately eight-times more selective towards the α2 adrenoceptor than clonidine.[5] In previous clinical studies, intravenous dexmedetomidine resulted in significant opioid sparing effects as well as a decrease in inhalational anaesthetic requirements.[6] In various animal studies, dexmedetomidine has been reported to enhance sensory and motor blockade along with increased duration of analgesia.[7–10] In humans, dexmedetomidine has also shown to prolong the duration of block and post-operative analgesia when added to local anaesthetic in various regional blocks.[11–14] Till date, no studies have compared dexmedetomidine with clonidine with respect to duration of block and post-operative analgesia. The current study was designed to test the hypothesis that dexmedetomidine when added as an adjuvant to local anaesthetic in supraclavicular brachial plexus block enhanced the duration of sensory and motor block, duration of analgesia and quality of block as compared with clonidine.
METHODS
After ethical committee approval and written informed consent, a double-blind randomized prospective clinical study was carried out on 60 American Society of Anaesthesiologist (ASA) Grade I and II patients of either sex, aged 18–60 years, undergoing various bony orthopaedic surgeries on the upper limb under supraclavicular brachial plexus block. The study was conducted in two groups of 30 patients each. The patients were randomly assigned using “slips in a box technique” to one of the following groups:
Group C: Bupivacaine 0.25% (35 cc) + clonidine 1 μg/kg
Group D: Bupivacaine 0.25% (35 cc) + dexmedetomidine 1 μg/kg
Patients on adrenoreceptor agonist or antagonist therapy, with known hypersensitivity to local anaesthetic drugs, bleeding disorders, uncontrolled diabetes mellitus, pregnant women and pre-existing peripheral neuropathy, were excluded from the study.
On arrival in the operation room, baseline heart rate, blood pressure and oxygen saturation were recorded. An intravenous line was secured in the unaffected limb and Ringer's lactate was started.
All the patients received brachial plexus block through the supraclavicular approach by an experienced anaesthesiologist different from the one assessing the patient intra- and post-operatively. Both were blinded to the treatment groups. Neural localization was achieved by using a nerve locator (Fisher and Paykel, New Zealand) connected to a 22 G, 50-mm-long stimulating needle (Stimuplex, Braun, Germany). The location end point was a distal motor response with an output lower than 0.5 mA in the median nerve region.
Following negative aspiration, 35 mL of a solution containing local anaesthetic combined with clonidine or dexmedetomidine as mentioned above was injected. A 3-min massage was performed to facilitate an even drug distribution.
Sensory block was assessed by the pin prick method. Assessment of sensory block was done at each minute after completion of drug injection in the dermatomal areas corresponding to median nerve, radial nerve, ulnar nerve and musculocutaneous nerve till complete sensory blockade. Sensory onset was considered when there was a dull sensation to pin prick along the distribution of any of the above-mentioned nerves. Complete sensory block was considered when there was complete loss of sensation to pin prick.
Sensory block was graded as-
Grade 0: Sharp pin felt
Grade 1: Analgesia, dull sensation felt
Grade 2: Anaesthesia, no sensation felt.
Assessment of motor block was carried out by the same observer at each minute till complete motor blockade after drug injection. Onset of motor blockade was considered when there was Grade 1 motor blockade. Peak motor block was considered when there was Grade 2 motor blockade. Motor block was determined according to a modified Bromage scale for upper extremities on a 3-point scale.[15]
Grade 0: Normal motor function with full flexion and extension of elbow, wrist and fingers
Grade 1: Decreased motor strength with ability to move the fingers only
Grade 2: Complete motor block with inability to move the fingers
The block was considered incomplete when any of the segments supplied by median, radial, ulnar and musculocutaneous nerve did not have analgesia even after 30 min of drug injection. These patients were supplemented with intravenous fentanyl (1 μg/ kg) and midazolam (0.02 mg/kg). When more than one nerve remained unaffected, it was considered a failed block. In this case, general anaesthesia was given intraoperatively. Patients were monitored for haemodynamic variables such as heart rate, blood pressure and oxygen saturation every 30 min after the block intraoperatively and every 60 min post-operatively. Sedation of patient was assessed by the Ramsay Sedation Score.[16] At the end of the procedure, quality of operative conditions were assessed according to the following numeric scale[12]:
Grade 4: (Excellent) No complaint from patient
Grade 3: (Good) Minor complaint with no need for the supplemental analgesics
Grade 2: (Moderate) Complaint that required supplemental analgesia
Grade 1: (Unsuccessful) Patient given general anaesthesia
Assessment of blood loss was done and fluid was administered as per the loss. Duration of surgery was noted.
The intra- and post-operative assessment was done by an anaesthesiologist who was unaware of the drug used. Patients were assessed for duration of analgesia as per a numeric rating scale of 0 to 10. The numeric rating scale was recorded post-operatively every 60 min till the score of 5. The rescue analgesia was given in the form of inj. diclofenac sodium (1.5 mg/kg) intramuscularly at the Neumeric Rating Scale of 5 and the time of administration was noted. All patients were observed for any side-effects like nausea, vomiting, dryness of mouth and complications like pneumothorax, haematoma, local anaesthetic toxicity and post-block neuropathy in the intra- and post-operative periods.
The duration of sensory block was defined as the time interval between the end of local anaesthetic administration and the complete resolution of anaesthesia on all nerves. The duration of motor block was defined as the time interval between the end of local anaesthetic administration and the recovery of complete motor function of the hand and forearm.
Statistical analysis
The data was analysed by SPSS version (Statistical Package for Social Sciences) software. Unpaired t-test was applied for demographic data, haemodynamic parameters, onset and duration of sensory and motor blockade and duration of analgesia. Fisher exact test was applied for assessment of quality of block. P-value was considered significant if <0.05 and highly significant if <0.001.
RESULTS
Eighty patients posted for upper limb surgeries were assessed for suitability to enroll in the study. Seven patients declined to participate in the study. Five patients were excluded as they were posted for soft tissue surgeries of the upper limb. Eight patients were excluded as they were found to be on beta blockers, anticoagulation drugs and had uncontrolled diabetes mellitus. The remaining 60 patients fulfilling the inclusion criteria were randomly assigned to one of the two groups. There was no protocol deviation pre-operatively and intraoperatively, except for one patient in group C who had to be given general anaesthesia for inadequate block.
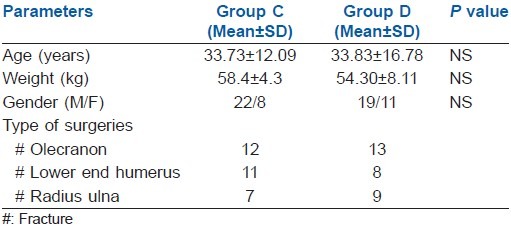
Both groups were comparable in terms of age, gender, weight and type of surgeries [Table 1] (P>0.001).
Table 1.
Patient characteristics
The baseline haemodynamic parameters were comparable in both groups. Significantly lower pulse rate was observed at 60, 90 and 120 min, but not less than 60 beats/min, in Group D as compared with Group C [Figure 1] (P<0.001).Systolic and diastolic blood pressure were found to be significantly lower than baseline from 30 to 120 min in Group D as compared with Group C (Graph II) (P<0.001). No treatment was required for this fall in blood pressure. The haemodynamic parameters were comparable at the end of 180 min. [Figure 2].
Figure 1.
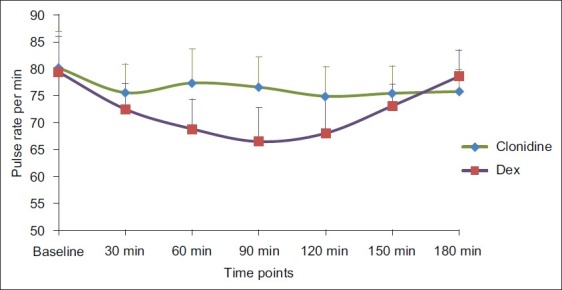
Comparison of pulse rate in both the groups
Figure 2.
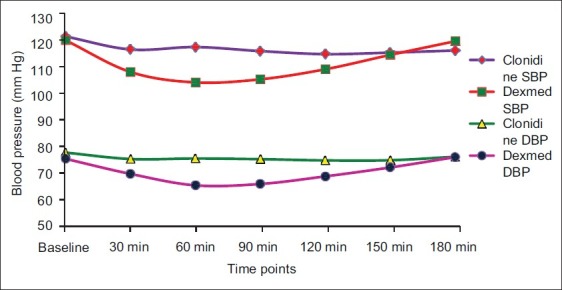
Comparison of systolic blood pressure and diastolic blood pressure in both the groups
Onset of sensory block was faster in Group D than in Group C, while onset of motor block was faster in Group C than in Group D, but the difference was not statistically significant [Table 2] (P>0.001).
Table 2.
Sensory and motor block onset time, block and analgesia durations in both groups
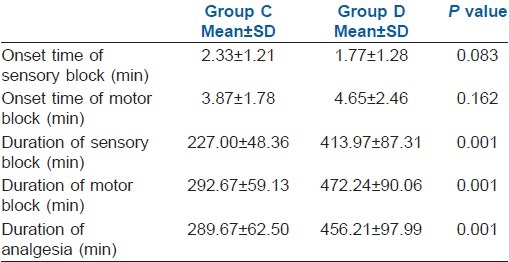
Duration of sensory block was 227.00±48.36 min in Group C as compared with 413.97±87.31 min in Group D. Statistically significant longer duration of sensory block was observed in Group D [Table 2 and Figure 3] (P=0.001).
Figure 3.
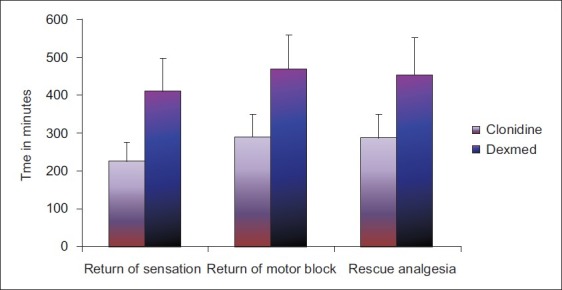
Comparison of duration of sensory block, motor block and analgesia in both the groups
The duration of motor block was 292.67±59.13 min in Group C as compared with 472.24±90.06 min in Group D. Again, duration of motor block was significantly longer in Group D [Table 2 and Figure 3] (P=0.001).
There was significant increase in duration of analgesia in Group D (456.12±97.99 min) as compared with Group C (289.67±62.50 min). The difference was statistically significant [Table 2 and Figure 3] (P=0.001)
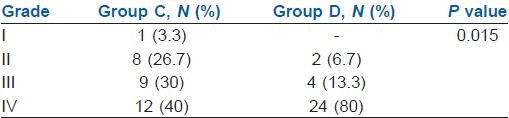
In Group D, 80% of the patients achieved Grade IV quality of block as opposed to 40% in Group C (P<0.05). There were a total 17 patients in Group C with Grade II and III block and six patients in Group D who required sedation or sedation with analgesia. One patient in Group C required general anaesthesia as the block was inadequate [Table 3].
Table 3.
Quality of block
No side-effects (nausea, vomiting, dry mouth) were reported during the first 24 h in the post-operative period in both the groups.
DISCUSSION
In this randomized, double-blinded trial, we compared dexmedetomidine and clonidine (α2 agonist) as an adjuvant to Bupivacaine in supraclavicular brachial plexus block, and found that there was a significantly increased duration of sensory and motor blockade in the dexmedetomidine group than in the clonidine group without any adverse effects.
Peripheral action of clonidine
Clonidine was initially used for its antihypertensive properties. The central actions are mediated through α2 adrenoceptors, which are situated at locus coeruleus and dorsal horn of spinal cord. But, specific peripheral effects of clonidine appear to be less obvious because α2 adrenoceptors are not present on the axon of the normal peripheral nerve.[4] There have been four proposed mechanisms for the action of clonidine in peripheral nerve blocks. These mechanisms are centrally mediated analgesia, α2 β adrenoceptor-mediated vasoconstrictive effects, attenuation of inflammatory response and direct action on peripheral nerve.[7] The direct action of clonidine on the nerve can be explained on the basis of a study conducted by Dalle et al. They proposed that clonidine, by enhancing activity-dependent hyperpolarisation generated by the Na/K pump during repetitive stimulation, increases the threshold for initiating the action potential causing slowing or blockage of conduction.[17] Kosugi et al. examined the effects of various adrenoceptor agonists including dexmedetomidine, tetracaine, oxymetazoline and clonidine, and also an α2 adrenoceptor antagonist (atipamezole) on compound action potential (CAP) recorded from frog sciatic nerve, and found that CAPs were inhibited by α2 adrenoceptor agents so that they are able to block nerve conduction.[10]
Popping et al. in their metaanalysis of randomized trials showed that the beneficial effect of clonidine on the duration of analgesia was observed with all tested local anaesthetics. They observed that the prolongation of motor block was higher when clonidine was added to bupivacaine as compared with ropivacaine. The least effect was noted with prilocaine.[4]
Peripheral action of dexmedetomidine
Dexmedetomidine and clonidine are both α2 selective agonists. It is possible that they work in a similar manner and may indicate a class effect.
A study by Brumett et al. showed that dexmedetomidine enhances duration of bupivacaine anaesthesia and analgesia of sciatic nerve block in rats without any damage to the nerve. The histopathological evaluation of these nerve axons and myelin were normal in both control and dexmedetomidine + bupivacaine groups.[7]
In an another study, perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolonged the duration of analgesia by blocking the hyperpolarisation-activated cation. This effect was reversed by a hyperpolarisation-activated cation channel enhancer but not by an α2 adrenoreceptor antagonist. This shows that the analgesic effect of peripheral perineural dexmedetomidine was caused by enhancement of the hyperpolarisation-activated cation current, which prevents the nerve from returning from a hyperpolarized state to resting membrane potential for subsequent firing.[9]
Kousugi et al. in their study found that high concentrations of dexmedetomidine inhibit CAPs in frog sciatic nerves without α2 adrenoceptor activation. Their result showed that dexmedetomidine reduced the peak amplitude of CAPs reversibly and in a concentration- dependent manner. This action was not antagonized by α2 adrenoceptor antagonists (i.e., yohimbine and atipamezole); rather, α2 antagonists reduced the CAP peak amplitude. Clonidine and oxymetazoline, two other α2 agonists, also inhibit CAPs. The maximum effect of clonidine was only 20%. On the other hand, adrenaline, noradrenaline and α1 agonist phenylephrine and beta agonist isoprenaline had no effect on CAPs.[10]
The efficacy of peripheral perineural dexmedetomidine added to bupivacaine and ropivacaine for sciatic nerve blocks in rats has been established.[8,9] The increase in duration of analgesia is dose dependent[9] and the effect is peripheral (i.e., not caused by centrally mediated or systemic analgesia).[8]
However all studies carried out so far to prove the peripheral action of α2 agonists were animal studies. There are very few human studies, i.e. greater palatine and axillary brachial plexus nerve blocks have subsequently demonstrated that increased duration of sensory blockade can be achieved by adding dexmedetomidine to bupivacaine and levobupivacaine, respectively.[13,14] Keeping these facts in mind, we decided to compare the action of two α2 agonists, i.e. clonidine and dexmedetomidine with bupivacaine in lesser concentration (0.25%), in peripheral nerve blocks so that by increasing the duration of analgesia with a single shot block we can achieve a longer duration of post-operative analgesia without significant clinical side-effects and hence we can avoid continuous catheterization.
Singelyn et al. reported that a minimum dose of clonidine (0.5 μg/kg) added to mepivacaine prolongs the duration of anaesthesia and analgesia after brachial plexus block. No added benefits were found with doses exceeding 1.5 μg/kg. The enhancing effect of a small dose of clonidine on lignocaine may be because of the evoked inhibition of C-fiber action potential. Therefore, we decided to use clonidine at a dose of 1 μg/kg in our study.[3]
Although dexmedetomidine has a α2/α1 selectivity ratio that is eight-times higher than that of clonidine, an equipotent comparative study of both the drugs in peripheral nerve block was not available at the time of our study. The dose selection was based on previous studies where dexmedetomidine 1 μg/kg and clonidine 1 μg/kg were used in Bier's block as an adjuvant to lignocaine.[18] After literature review, we found that dexmedetomidine and clonidine had peripheral action, which may be useful in using a lesser concentration of local anaesthetic (0.25%) to prolong the block with adequate anaesthesia. This in turn may be beneficial in high-risk patients.
In our study, we compared the addition of clonidine (Group C 1 μg/kg) and dexmedetomidine (Group D 1 μg/kg) to bupivacaine in supraclavicular brachial plexus block. The result of our study shows that all patients in both groups were comparable with respect to demographic profile, duration of surgery and type of surgery. With these doses, we had stable haemodynamics in patients except significant lower pulse rate in Group D at 60, 90 and 120 min as compared with Group C, but not less than 60 beats/min.
Esmaoglu et al. added dexmedetomidine to levobupivacaine for axillary brachial plexus block and showed that it shortens the onset time of both sensory and motor block, prolongs the duration of block and the duration of post-operative analgesia.[13] This may be because peripheral α2 agonist produces analgesia by reducing release of norepinephrine, leading to α2 receptor-independent inhibitory effects on nerve fiber action potentials.[12,13] However, in our study, we found that onset of sensory block was a little faster with Group D as compared with Group C, but it was statistically insignificant, while onset of motor block was a little longer in Group D but again not significant statistically. The duration of analgesia in Group D was longer than in Group C, and it was statistically significant.
The concern of prolongation of motor block. was minimal patient discomfort on movement in the post-operative period.
Memis et al. in their study showed that addition of dexmedetomidine to lignocaine for intravenous regional anaesthesia improves both the quality of anaesthesia as well as intraoperative and post-operative analgesia.[12] In our study, the quality of block in 80% of the patients in Group D was grade IV, i.e. excellent block without any supplementary sedation or analgesia, while 40% in Group C achieved grade IV quality. This improved quality of block might be the result of various mechanisms of nerve conduction block such as hyperpolarisation,[4] decreased CAP[10] and inhibition of voltage gate of sodium pump.
None of the patients in Group D required sedation intraoperatively and they were comfortable throughout the surgery with arousable sedative effects. This can be explained on the basis that some amount of systemic absorption of drug could be present.[4] As α2 agonists produce sedation by central action, they produce inhibition of substance P release in the nociceptive pathway at the level of the dorsal root neuron and by activation of α2 adrenoreceptor in locus coeruleus.[18]
The major limitations of our study are that we did not use ultrasound-guided blocks because of unavailability at the time of our study; this could have helped us to lower dosages and volumes of local anaesthetic. In spite of an intensive search of the published literature, we were unable to identify an ideal scale for assessment of quality of block achieved. While the higher cost of dexmedetomidine can be suggested as a reason for preference for clonidine, the increased requirement of supplementary analgesia and sedation with clonidine may balance this. We admit that further studies to determine the cost-effectiveness of the drug are necessary.
From this study, we would like to suggest that dexmedetomidine can be safely used with local anaesthetic in peripheral nerve blocks; however, further trials to determine the exact dose and effect of neurotoxicity on the human nerve are required.
CONCLUSION
To conclude, we would like to state that dexmedetomidine prolongs the duration of sensory and motor block and enhances the quality of block as compared with clonidine when used as an adjuvant to Bupivacaine in peripheral nerve block.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Damien B, Murhy, Collin JL, Cartney, Vincent WS. Novel analgesic adjuvants for brachial plexus block: A systemic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Elliott S, Eckersall S, Fliqelstone L. Does addition of clonidine affect duration of analgesia of Bupivacaine in inguinal hernia repair. Br J Anaesth. 1997;79:446–9. doi: 10.1093/bja/79.4.446. [DOI] [PubMed] [Google Scholar]
- 3.Singelyn FJ, Gouveineur J, Robert A. A minimum dose of clonidine added to mepivacaine prolongs duration analgesia after brachial plexus block. Anesth Analg. 1996;83:1046–50. doi: 10.1097/00000539-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Popping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anaesthetic for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology. 2009;111:406–15. doi: 10.1097/ALN.0b013e3181aae897. [DOI] [PubMed] [Google Scholar]
- 5.Raimo V, Juha M, Veijo S, Leena N, Virtanen R. Characterisation of selectivity, specificity and potency of medetomidine as α2 adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 6.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with Bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 9.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyper polarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potential in frog sciatic nerve without α2 adrenoceptor activation. Br J Pharmacol. 2010;160:1662–76. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanazi GE, Aouad MT, JAbbour- Khoury SL, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effects of low dose Dexmedetomidine or clonidine on characteristics of spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 12.Memis D, Turan A, Karamanlioglu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lignocaine for IVRA. Anesth Analg. 2004;98:835–40. doi: 10.1213/01.ane.0000100680.77978.66. [DOI] [PubMed] [Google Scholar]
- 13.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anaesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 14.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to Bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar DJ, Khurana G, Chaudhary A, Sharma J P. A comparative study on the effects of adding fentanyl and buprenorphine to local anaesthetics in brachial plexus block. Journal of Clinical and Diagnostic Research. 2010;4(6):3337–43. [Google Scholar]
- 16.Ramsay MA, Savage TM, Simpson BR, Godwin R. Controlled sedation with alphaxolone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P. Inhibition of the I (h) current in isolated peripheral nerve: A novel mode of peripheral antinociception? Muscle Nerve. 2001;24:254–61. doi: 10.1002/1097-4598(200102)24:2<254::aid-mus110>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Abosedira MA. Adding clonidine or dexmedetomidine to lignocaine during Biers block: A comparative study. J Med Sci. 2008;8:660–4. Doi:10.3923/jms.2008.660-664. [Google Scholar]


