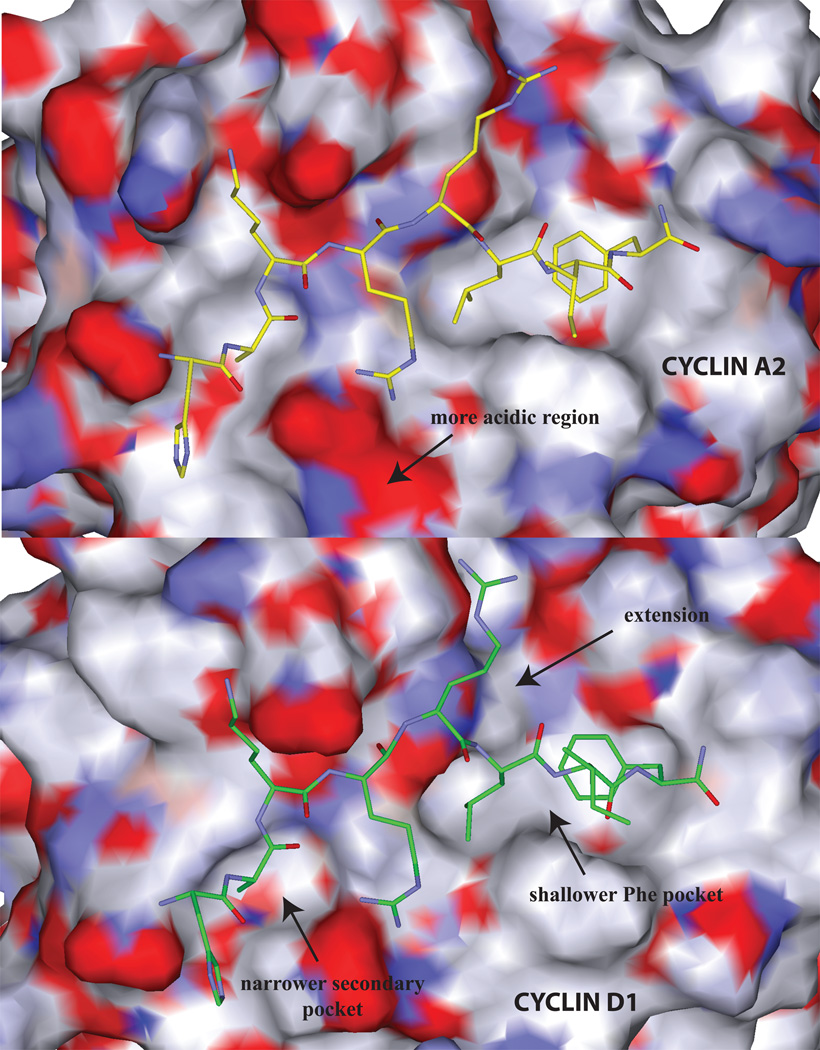
Figure 2.
Comparison of the solvent accessible surface of the cyclin grooves of A2 (top panel) and D1 (bottom). The following regions displaying structural differences in cyclin binding determinants are labeled: 1) the top panel illustrates the more acidic region of cyclin A compared to cyclin D1 which results in differential interactions with Arg4. 2) the primary hydrophobic extension of cyclin D1 (top right), 3) the shallower cyclin D1 primary hydrophobic pocket (the Leu214 – Val60 interchange; bottom right) and 4) the narrower cyclin D1 secondary hydrophobic pocket (left). Each of these structural features explains the differential requirements for cyclin groove inhibitor binding.