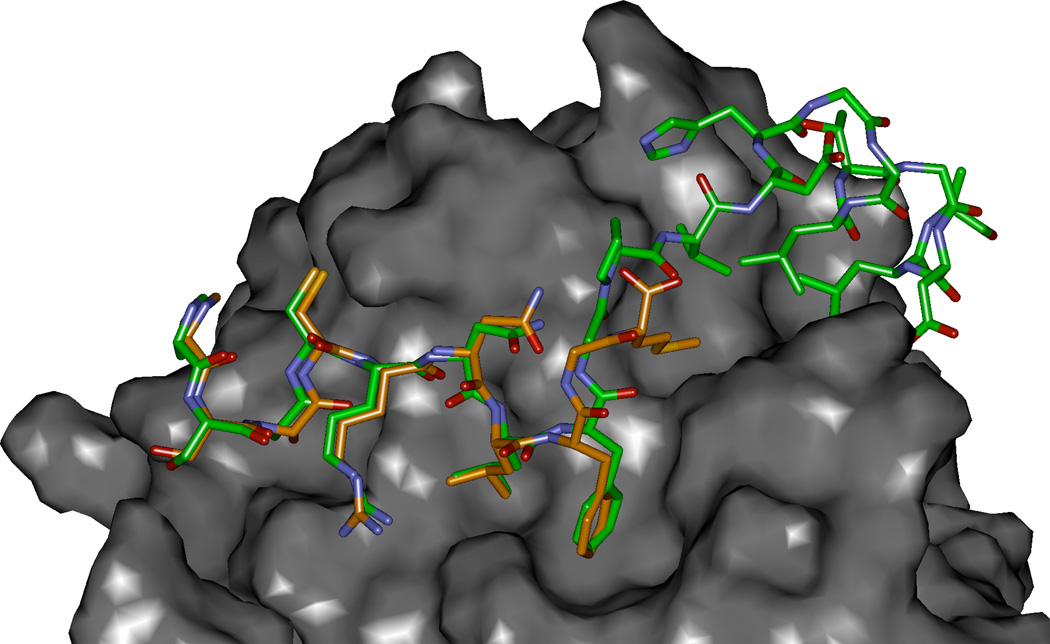
Figure 3.
Modeled complex of p27 residues 25–49 (green carbons) with Cyclin D1 (2W96) overlayed with SAKRNLFGM. The P35 and V36 interacting site on cyclin D1 is the region shown to provide a more extensive hydrophobic pocket than in the cyclin A2 context and which was exploited by methionine substitution (Met is the C-terminal residue in the p27 peptide colored with orange carbon atoms).