Abstract
Polycystic ovary syndrome (PCOS) recently has been identified as a risk factor associated with type 2 diabetes. However, the evidence derives from cross-sectional observational studies, retrospective studies, or short-term prospective studies. This long-term prospective study of a large cohort of women with PCOS, followed from youth to middle age, aimed at estimating, for the first time, the incidence and potential predictors of type 2 diabetes in this population. A total of 255 women with PCOS were followed for at least 10 years (mean follow-up 16.9 years). Six women were patients with diabetes at baseline, and another 42 women developed type 2 diabetes during the follow-up. The incidence rate of type 2 diabetes in the study population was 1.05 per 100 person-years. The age-standardized prevalence of diabetes at the end of follow-up was 39.3%, which is significantly higher with respect to that of the general Italian female population of a similar age (5.8%). The likelihood of developing type 2 diabetes significantly increased as BMI, fasting glucose, and glucose area under the curve at baseline increased and significantly decreased as sex hormone–binding globulin (SHBG) levels at follow-up increased. This study demonstrates that the risk of type 2 diabetes is markedly elevated in middle-aged women with PCOS and suggests including BMI, glucose, and SHBG-circulating levels in the risk stratification.
The polycystic ovary syndrome (PCOS) is a common female disorder defined by the combination of hyperandrogenism, menstrual and ovulatory alterations, and polycystic ovarian morphology (1). However, the consequences of PCOS extend beyond the reproductive axis. Most women with PCOS, particularly those presenting with overweight or obesity (2), do in fact have insulin resistance and compensatory hyperinsulinemia (3,4), partly attributed to intrinsic insulin resistance mechanisms (5–7). Insulin resistance is a known key factor in the development of type 2 diabetes (8). Several studies have demonstrated that type 2 diabetes occurs with increased frequency in women with PCOS (9,10), so that PCOS recently has been identified as a significant nonmodifiable risk factor associated with type 2 diabetes by the International Diabetes Federation and by the American Diabetes Association (11,12). However, this evidence derives from cross-sectional observational studies, retrospective studies, or short-term prospective studies (9). To date, only one prospective study has been performed in women with PCOS for a relatively long time (8-year time span); unfortunately, this study was limited by the use of fasting glucose to diagnose diabetes (13). No other long-term prospective study has been performed so far, and, therefore, we currently are unable to quantify the lifetime incidence of type 2 diabetes in women with PCOS and the risk factors for type 2 diabetes associated with the syndrome. To provide such information, we performed this long-term prospective study of a large cohort of well-characterized women with PCOS followed from youth to middle age.
RESEARCH DESIGN AND METHODS
This study had an observational longitudinal design. Italian women with PCOS were evaluated at baseline and for at least 10 years in order to estimate the incidence and potential predictors of type 2 diabetes in middle age.
At the first examination (between 1978 and 1999), all women entering our unit were screened for the presence of PCOS, and the diagnosis was made by a trained physician according to National Institutes of Health criteria (14). For all women evaluated before 1992, the diagnosis of PCOS was revaluated according to the same National Institutes of Health criteria.
At baseline, women were excluded if they had Cushing’s syndrome or an androgen-secreting neoplasm, hyperprolactinemia, congenital adrenal hyperplasia, thyroid disease, or other causes of amenorrhea, including premature ovarian failure. At baseline, all women were untreated.
During 2009, all women previously diagnosed with PCOS were asked to participate (by phone and/or letter) in the follow-up examination, in which the same information and tests as the first visit were performed, using the same methodology.
A structured interview was carried out with all participants, collecting data on demographic status, smoking, drug therapy, and familial and personal history of diseases (family history was considered positive when at least one first-degree relative was affected by a certain disease).
Women underwent a physical examination that included anthropometric (height, weight, and waist circumference) and blood pressure measurement by a physician. Height was measured without shoes and rounded to the nearest 0.5 cm; weight was measured without clothes, and BMI was computed as weight (in kilograms) divided by the square of height (in meters). Waist circumference was measured with subjects standing, using a 1-cm-wide metal measuring tape, according to World Health Organization criteria (15). Blood pressure was measured twice in the supine position, in the morning, after at least 3 min of rest before any measurement, and the mean of the two recordings was used. Women were instructed not to drink coffee or water in the 3 h before the examination.
Fasting blood samples also were taken in order to evaluate the levels of leutinizing hormone, follicle-stimulating hormone, 17β-estradiol, testosterone, and sex hormone–binding globulin (SHBG) and to compute the free androgen index, following the methodology described by Vermeulen et al. (16). Blood samples were taken during the first week after spontaneous bleeding initiation in women with normal menses or mild oligomenorrhea and after withdrawal bleeding induced by a course of oral medroxyprogesterone acetate in women with severe oligomenorrhea or amenorrhea. All samples were centrifuged immediately and stored at −80°C until assayed (in a single, centralized, and certified laboratory).
Finally, after blood samples had been obtained, a 75-g oral glucose tolerance test was performed, taking blood samples while fasting and 30, 60, 90, 120, and 180 min after glucose ingestion (Curvosio; Sclavo, Cinisello Balsamo, Italy). The response of glucose at 120 min was used to diagnose diabetes according to American Diabetes Association criteria (8). All participants provided written, informed consent at both assessments, and the study protocol was approved by the local ethics committee.
Outcomes and data analysis.
The primary outcome of the study was the incidence of type 2 diabetes in women with PCOS. Both the cumulative incidence and the incidence rate (expressed as the number of cases of diabetes per 100 person-years) were computed. It was not possible to standardize the incidence rate of the sample using population data because we could not find reliable 15-year incidence data, stratified by age, of a general Italian population (to use as a control group). To provide some form of comparison, however, we calculated the standardized prevalence of diabetes at the end of follow-up and compared it with the standardized prevalence of the general Italian female population of the same age. We used direct standardization based upon data from the Italian Institute of Statistics, updated in 2008 (end of follow-up data) (http://www3.istat.it/dati/pubbsci/documenti/documenti2008.html). The following age classes were used for standardization: 0–14, 15–24, 25–34, 35–44, 45–54, 55–64, and 65–74 years.
As a secondary analysis, we also investigated potential predictors of type 2 diabetes in women with PCOS. Cox proportional hazards analysis, restricted to women without diabetes at baseline (n = 249), was used to calculate the adjusted relative hazards of type 2 diabetes by each recorded variable, including drug treatment throughout the follow-up period. Stochastic level of entry into the model was set at 0.10, and interaction terms were explored for all variables in the final model. Covariates were categorized or log transformed if needed (normality being checked with the Shapiro-Wilk test), and interaction terms and multicollinearity were explored for all significant variables in the final model. A minimum events-to-variable ratio of 10 was maintained in multivariate modeling to avoid overfitting, and the Schoenfeld test was performed to check the validity of proportional hazards assumption (17). Glucose response to the oral glucose tolerance test was expressed as the area under the curve (AUC), which was calculated by the trapezoidal method. Statistical significance was defined as a two-sided P value <0.05 for all analyses, which were performed using Stata 10.1 (Stata, College Station, TX).
RESULTS
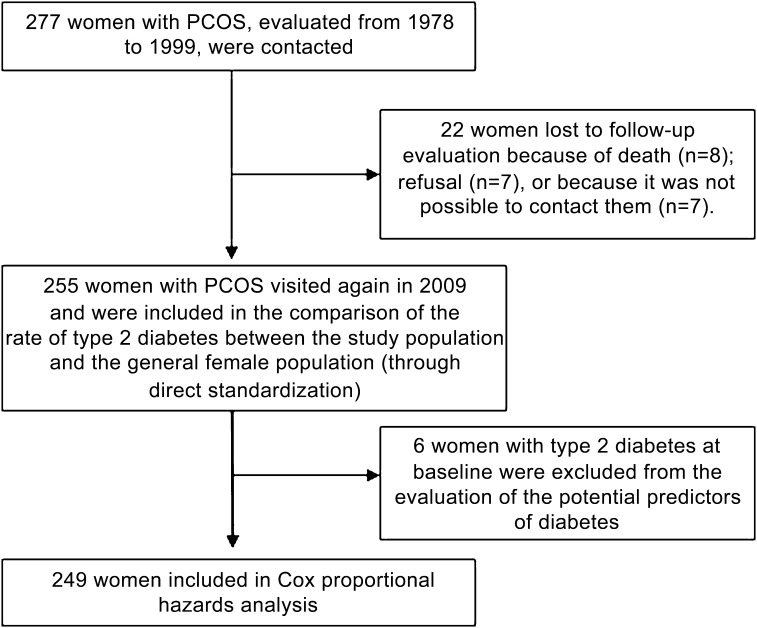
A total of 277 consecutive women with PCOS initially were evaluated in our unit from 1978 to 1999. All were contacted in 2009 for the follow-up assessment, but data on 22 women (7.9%) could not be collected (Fig. 1) (8 women were deceased [reasons are reported in boxed text], 7 women refused to participate [2 because they had just given birth and 5 because they were not interested], and it was not possible to contact the remaining 7 women).
FIG. 1.
Flow of the participants.
For patients no longer living (8 of 277), a primary relative was queried as to the cause of death. Four patients died of cancer (one of ovarian cancer at age 51 years, one of a brain tumor at age 38 years, one of leukemia at age 36 years, and one of unspecified cancer at age 42 years). The remaining four women died of pulmonary embolism at age 43 years, of alcoholic liver disease at age 41 years, of acute myocardial infarction at age 64 years, and of a road accident at age 32 years.
Therefore, 255 women (92% from northern Italy, 2% from central Italy, and 6% from southern Italy) were re-evaluated in 2009, and their data were used in the comparison between the rates of type 2 diabetes of women with PCOS and the general female population. Most of the women with PCOS (n = 226) were being regularly followed-up at our unit (one to two check-ups every 1–2 years), and this may explain the very high participation rate.
During the follow-up period, 163 subjects had been on oral contraceptive treatment for at least 1 year, 72 subjects were on metformin (1,500 or 1,700 mg/day), 29 subjects were on flutamide (125 or 250 mg/day), 22 subjects were on antihypertensive drugs, and 4 subjects were on statins (atorvastatin or simvastatin). Oral contraceptives or flutamide were started in subjects with clinically significant hyperandrogenism (hirsutism, acne, or alopecia), whereas metformin was started in subjects with a dominant dysmetabolic profile, particularly insulin resistance and hyperinsulinemia. The mean period of treatment with oral contraceptives during the follow-up was 5.4 ± 5.9 years (range 1–27), and with metformin it was 1.3 ± 2.8 years (1–14). The progestin component of oral contraceptives was cyproterone acetate in 88 (54%) women, gestoden in 45 (28%) women, desogestrel in 20 (12%) women, levonorgestrel in 5 (3%) women, and drospirenone in 5 (3%) women. For patients still on treatment at the follow-up examination, oral contraceptives or flutamide were discontinued 3 months before blood collection, whereas metformin or statins were discontinued 3 weeks before blood collection.
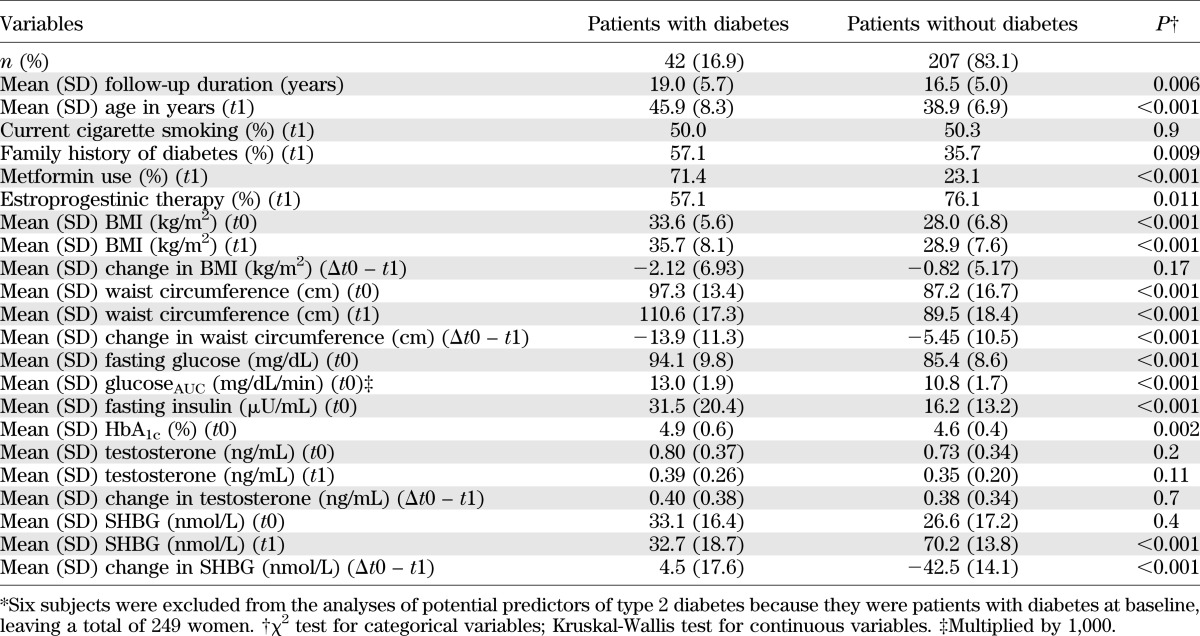
The baseline characteristics of the sample and of the 22 subjects lost to follow-up are reported in Table 1. No differences were observed in any parameter, with the exception of leutinizing hormone blood levels, which were significantly higher among participants (P = 0.032).
TABLE 1.
Baseline characteristics of the sample and of subjects lost to follow-up
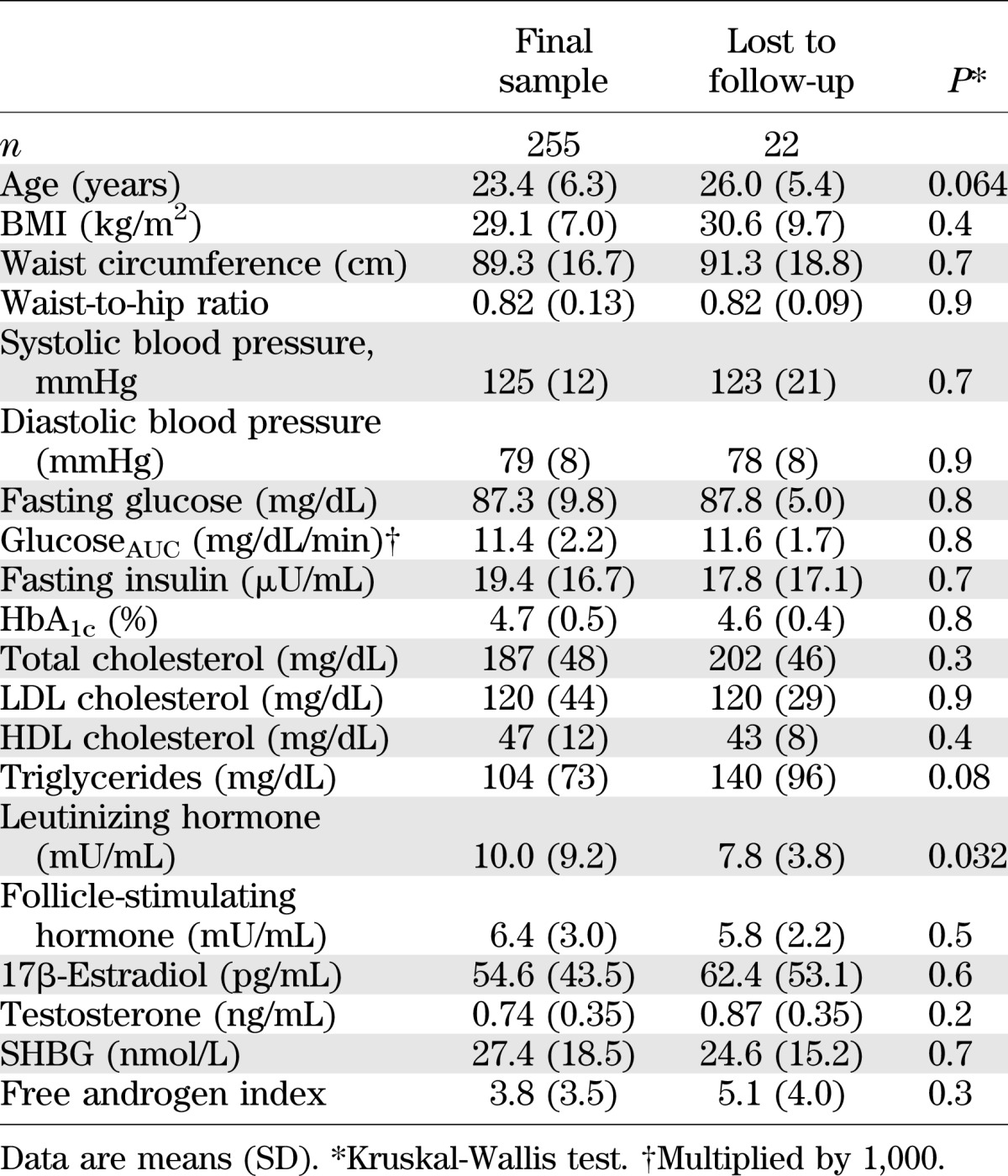
Six women were diabetic at baseline (2.2%), and another 42 women developed type 2 diabetes during the follow-up (cumulative incidence: 16.9%, incidence rate: 1.05 per 100 person-years [95% CI 0.75–1.41]). At the end of follow-up, the age-standardized prevalence rate of type 2 diabetes of the study population was 39.3% (95% CI 33.4–45.4), which was significantly higher than that of the general population (5.8% [3.3–9.3]; P < 0.001). The characteristics of the 42 women who developed type 2 diabetes during the follow-up, and those who remained free of disease are compared in Table 2. The mean follow-up was slightly but significantly longer in diabetic women with respect to nondiabetic women, and, accordingly, at the follow-up assessment, diabetic women were significantly older. Diabetic women had significantly higher BMI and larger waist circumferences both at baseline and at the end of the follow-up, but, unlike BMI, changes in waist circumference were sig-nificantly higher in diabetic women with respect to nondiabetic women. Moreover, diabetic women had higher fasting glucose (although in the normal range) and insulin levels and higher glucoseAUC at baseline but lower SHBG levels at follow-up. Finally, first-degree familiarity for type 2 diabetes was higher in diabetic women with respect to nondiabetic women. Diabetic women also had a higher prevalence of treatment with metformin during the follow-up period and a lower prevalence of treatment with oral contraceptives with respect to women free of disease.
TABLE 2.
Selected characteristics of the sample classified by type 2 diabetes, at baseline (t0) and at follow-up (t1)*
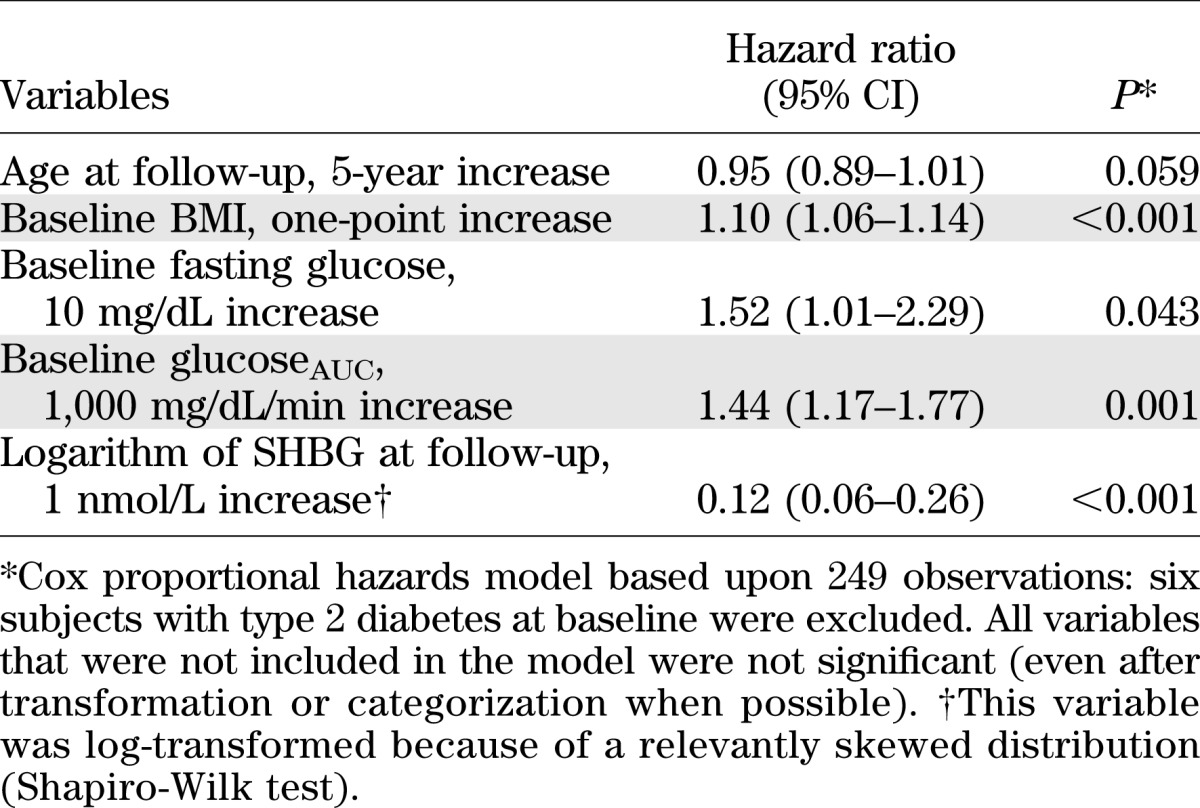
Of all the variables that were significantly associated with type 2 diabetes at the univariate analysis, only four were confirmed as independent predictors at multivariate analysis, in which the six women with type 2 diabetes at baseline were not included (Table 3). All results were adjusted for age at follow-up, although they only were borderline significant. The likelihood of developing type 2 diabetes significantly increased as BMI, fasting glucose, and glucoseAUC increased at baseline. Conversely, a higher SHBG level at follow-up was associated with a lower probability of developing type 2 diabetes.
TABLE 3.
Adjusted hazard ratios of type 2 diabetes according to selected variables
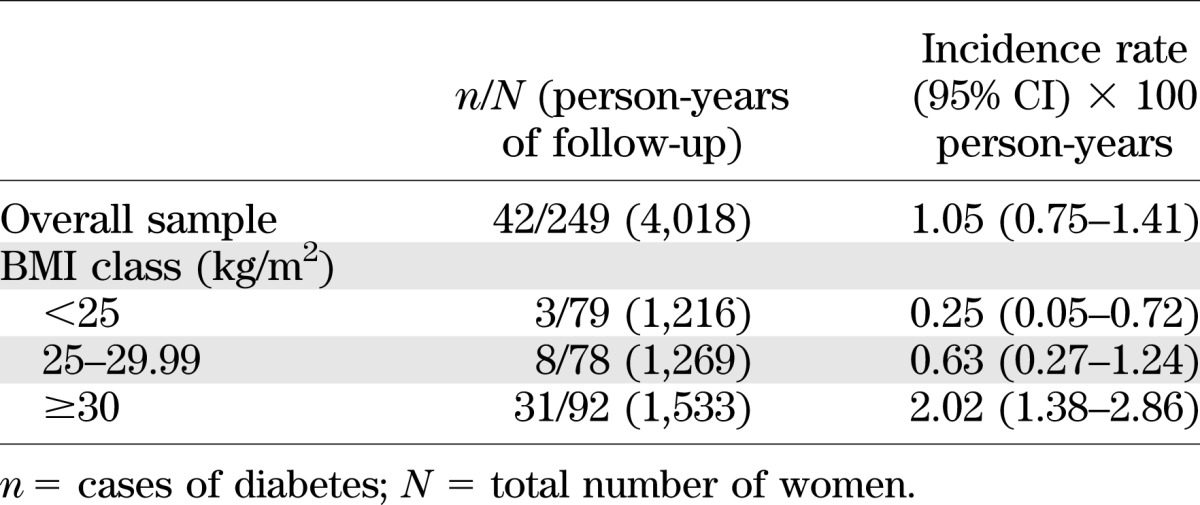
Incidence rates of type 2 diabetes stratified by class of BMI at baseline revealed that the absolute risk of diabetes increased steadily with BMI and was particularly high for BMI ≥30 kg/m2 (2.02 per 100 person-years, for an incidence rate ratio equal to 4.44 vs. nonobese women; P < 0.001) (Table 4) (results were very similar using BMI at the end of follow-up; data not shown).
TABLE 4.
Crude incidence rate of type 2 diabetes in the sample, overall and according to BMI class
DISCUSSION
Incidence data for type 2 diabetes in women with PCOS were derived from studies with several methodological limitations, including small sample size and, for most of them, a limited time of follow-up (13,18,19). This implies a relatively young age of the study population and, probably, a not full expression of adverse metabolic functioning of the PCOS. However, all these studies reported rapid conversion rates from normal glucose tolerance or impaired glucose tolerance to type 2 diabetes, often exceeding 15% per year, suggesting that in women with PCOS type 2 diabetes may occur earlier than expected in comparison with the general population. The long-term longitudinal design of the current study allowed us to confirm that the incidence of type 2 diabetes in Italian women with PCOS is 2.6 times higher than that of the general female population (1.05 vs. 0.40 per 100 person-years, estimated from the data of a recently published large, population-based Italian study [20]). Moreover, as a cumulative effect of such a higher incidence, the age-standardized prevalence of type 2 diabetes in women with PCOS at middle age is 6.8 times higher than that of the general female population of a similar age. Also, in this work we were able to define several predictive factors of the development of type 2 diabetes in these women. In fact, higher BMI, higher fasting glucose levels, and higher glucose response to the glycemic load at baseline proved to be independently associated with an increased risk of type 2 diabetes, whereas higher plasma levels of SHBG at follow-up proved to be associated with a lower risk of type 2 diabetes.
Obesity is common among women with PCOS (2), and a stepwise increase in prevalence of glucose intolerance as BMI increases has been described in cross-sectional studies performed in women with this disorder (21). Thus, it was expected (and in line with the literature available on the general population [22,23]) that high baseline levels of BMI and of glucose were predictive of development of type 2 diabetes in women with PCOS. Some authors have suggested that obesity, through still unknown mechanisms, acts in concert with intrinsic defects in insulin action (24,25) or insulin secretion (26,27) to enhance the risk of type 2 diabetes in PCOS. However, given the strong association between higher BMI with diabetes even in the general population (28), another important question is whether PCOS poses an intrinsic risk for the development of diabetes, whether there may be a synergistic effect between PCOS and high BMI, or finally whether the observed association between PCOS and diabetes is entirely attributable to obesity, which is so frequent in women with PCOS. This study does not provide a satisfactory answer to this question because of a lack of an ad hoc control population. However, when incidence rates of diabetes were stratified by class of BMI at baseline, the absolute risk of diabetes increased steadily with BMI and was particularly high for obese women (2.02 per 100 person-years, for an incidence rate ratio equal to 4.44 vs. other women; P < 0.001) (Table 4) (results were very similar using BMI at the end of follow-up; data not shown). This finding is in agreement with the observed increase of diabetes risk by BMI in the general female population (28) and suggests that BMI, and in particular obesity, may be crucial for the development of type 2 diabetes in women with PCOS.
An interesting finding of the current study was the association between higher levels of SHBG at follow-up and the lower risk of type 2 diabetes in the study population. These data are consistent with most of the results from prospective studies performed in the general population (29). There is increasing evidence that hyperandrogenemia, per se, may reflect underlying metabolic dysfunction and a tendency toward glucose intolerance in women (30,31). Therefore, the first potential explanation of this association is that SHBG may influence the occurrence of type 2 diabetes by modulating the biologic effects of free testosterone on peripheral insulin sensitive tissues. However, it also is possible that high levels of SHBG may contribute directly (by still unknown mechanisms) to protect against the development of type 2 diabetes (29). Although insulin is the major negative factor in the control of SHBG synthesis in the liver (2), the persistence of the association between SHBG and diabetes after adjustment for baseline fasting insulin levels in the multivariate analysis suggests that insulin itself does not entirely account for the predictive value of SHBG, according to what was previously shown in the general female population (32). Similar conclusions can be reached with oral contraceptive treatment that did not enter into the Cox proportional hazards analysis, despite the significantly higher prevalence of oral contraceptive users in nondiabetic women with respect to diabetic women and the knowledge that 3 months discontinuation of oral contraceptives may not be sufficient to erase the prolonged stimulatory effect of oral contraceptives on SHBG synthesis (33). In addition, we cannot exclude the possibility that low SHBG levels in diabetic subjects reflect other coexisting diseases, such as hepatic steatosis (34), not investigated in the current study, or blood glucose levels themselves, as recently suggested by Selva et al. (35). In this study, the authors found that the expression of SHBG in hepatocytes were directly suppressed by either monosaccharide-induced hepatic lipogenesis, which is involved in the development of fatty liver, and by fructose or glucose, independent of insulin, via a downregulation of the hepatocyte nuclear factor 4α, a transcriptional factor that has been associated with a form of maturity-onset diabetes of the young.
Findings on the impact of treatments during the follow-up period deserve some comments. Given the widespread idea that metformin administration could prevent or delay the development of type 2 diabetes in PCOS (10,36), an unexpected result of the current study was that a relatively long-term concurrent metformin use during the follow-up was associated with an increased incidence of type 2 diabetes (40.3% among metformin users vs. 7.1% among nonusers). However, the lack of a case-control design and the fact that metformin was started in subjects with a more compromised metabolic state, therefore intrinsically more at risk for developing type 2 diabetes, imply caution in drawing any conclusion. In addition, considerable variability in the metabolic responses to metformin has been observed in women with PCOS, attributably to several potential factors such as different doses of the drug and genetic background (37), which were not considered in the current study.
An interesting finding was related to the long-term use of oral contraceptives, which had no significant impact on the development of type 2 diabetes. Although the data are biased by the fact that oral contraceptives were administered to women with PCOS with more severe clinical hyperandrogenism, they are in agreement with what was previously observed in healthy women (38,39).
Although the strength of our study is the prospective design and the long period of follow-up, the absence of a non-PCOS control group is nonetheless a major limitation, although we extrapolated a comparison with the Italian data on the prevalence of type 2 diabetes in the general female population. Also, the use of the general Italian population as the control group might be a source of bias related to geography, as we enrolled most participants from northern Italy. However, the prevalence of diabetes seems to be relatively homogeneous across Italy (40). Thus, if a bias as a result of the geography of the study site occurred, it is likely to be small.
In conclusion, this study gives consistency to available data in demonstrating that the risk of type 2 diabetes is markedly elevated in women with PCOS in middle-age and therefore reinforces the need for routine screening of PCOS for diabetes over time. In addition, these data provide further clinical insights in support of targeting BMI, glucose (fasting and after glucose ingestion), and SHBG circulating levels in risk stratification and intervention for the maintenance of glucose homeostasis. Taking into account the limits of this study, additional long-term prospective case-control studies are warranted to support these suggestions.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
A.G. contributed to acquisition, revision, and interpretation of data and drafted, critically revised, wrote, and approved the final version of the manuscript. L.P., P.A., U.P., and C.P. contributed to data acquisition, critically revised the manuscript for intellectual content, and approved the final version of the manuscript. L.M. performed statistical analysis of the data and drafted, critically revised, wrote, and approved the final version of the manuscript. R.P. contributed to revision and interpretation of data and drafted, critically revised, wrote, and approved the final version of the manuscript. R.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47 [DOI] [PubMed] [Google Scholar]
- 2.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002;26:883–896 [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800 [DOI] [PubMed] [Google Scholar]
- 4.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev 1999;20:535–582 [DOI] [PubMed] [Google Scholar]
- 5.Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril 2008;89:1039–1048 [DOI] [PubMed] [Google Scholar]
- 6.Ciaraldi TP, Morales AJ, Hickman MG, Odom-Ford R, Olefsky JM, Yen SS. Cellular insulin resistance in adipocytes from obese polycystic ovary syndrome subjects involves adenosine modulation of insulin sensitivity. J Clin Endocrinol Metab 1997;82:1421–1425 [DOI] [PubMed] [Google Scholar]
- 7.Corbould A, Kim YB, Youngren JF, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 2005;288:E1047–E1054 [DOI] [PubMed] [Google Scholar]
- 8.Puavilai G, Chanprasertyotin S, Sriphrapradaeng A; World Health Organization Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. Diabetes Res Clin Pract 1999;44:21–26 [DOI] [PubMed] [Google Scholar]
- 9.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2010;16:347–363 [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson J, Millward A, Stenhouse E, Pinkney J. Type 2 diabetes and cardiovascular disease in polycystic ovary syndrome: what are the risks and can they be reduced? Diabet Med 2010;27:498–515 [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451–463 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Screening for type 2 diabetes. Diabetes Care 2004;27:11–14 [Google Scholar]
- 13.Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep 2006;6:77–83 [DOI] [PubMed] [Google Scholar]
- 14.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Polycystic Ovary Syndrome. Dunaif A, Givens JR, Haseltine FP, Merriam GR, Eds. Boston, Blackwell Scientific Publications, 1992, p. 377–384. [Google Scholar]
- 15.World Health Organization. Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity. Geneva, World Health Org., 1997 [WHO/NUT/NCD/98.1] [PubMed] [Google Scholar]
- 16.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Survival Analysis. New York, John Wiley & Sons, 1999. [Google Scholar]
- 18.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999;22:141–146 [DOI] [PubMed] [Google Scholar]
- 19.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 2005;90:3236–3242 [DOI] [PubMed] [Google Scholar]
- 20.Monesi L, Baviera M, Marzona I, et al. Prevalence, incidence and mortality of diagnosed diabetes: evidence from an Italian population-based study. Diabet Med 2012;29:385–392 [DOI] [PubMed] [Google Scholar]
- 21.Legro RS. Diabetes prevalence and risk factors in polycystic ovary syndrome. Curr Opin Endocrinol Diabetes 2002;9:451–458 [Google Scholar]
- 22.Bloomgarden ZT. The American Diabetes Association’s 57th Annual Advanced Postgraduate Course: diabetes risk, vitamin D, polycystic ovary syndrome, and obstructive sleep apnea. Diabetes Care 2011;34:e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501–513 [DOI] [PubMed] [Google Scholar]
- 24.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–1174 [DOI] [PubMed] [Google Scholar]
- 25.Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab 1992;75:577–583 [DOI] [PubMed] [Google Scholar]
- 26.Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SSC. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab 1996;81:2854–2864 [DOI] [PubMed] [Google Scholar]
- 27.Dunaif A, Finegood DT. β-Cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1996;81:942–947 [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–797 [DOI] [PubMed] [Google Scholar]
- 29.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 31.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007;50:2076–2084 [DOI] [PubMed] [Google Scholar]
- 32.Bonnet F, Balkau B, Malécot JM, et al. ; DESIR Study Group Sex hormone-binding globulin predicts the incidence of hyperglycemia in women: interactions with adiponectin levels. Eur J Endocrinol 2009;161:81–85 [DOI] [PubMed] [Google Scholar]
- 33.Panzer C, Wise S, Fantini G, et al. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: a retrospective study in women with sexual dysfunction. J Sex Med 2006;3:104–113 [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev 2008;29:939–960 [DOI] [PubMed] [Google Scholar]
- 35.Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest 2007;117:3979–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomba S, Falbo A, Zullo F, Orio F., Jr Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 2009;30:1–50 [DOI] [PubMed] [Google Scholar]
- 38.Rimm EB, Manson JE, Stampfer MJ, et al. Oral contraceptive use and the risk of type 2 (non-insulin-dependent) diabetes mellitus in a large prospective study of women. Diabetologia 1992;35:967–972 [DOI] [PubMed] [Google Scholar]
- 39.Chasan-Taber L, Willett WC, Stampfer MJ, et al. A prospective study of oral contraceptives and NIDDM among U.S. women. Diabetes Care 1997;20:330–335 [DOI] [PubMed] [Google Scholar]
- 40.Gnavi R, Karaghiosoff L, Balzi D, et al. Diabetes prevalence estimated using a standard algorithm based on electronic health data in various areas of Italy. Epidemiol Prev 2008;32(Suppl.):15–21 [PubMed] [Google Scholar]