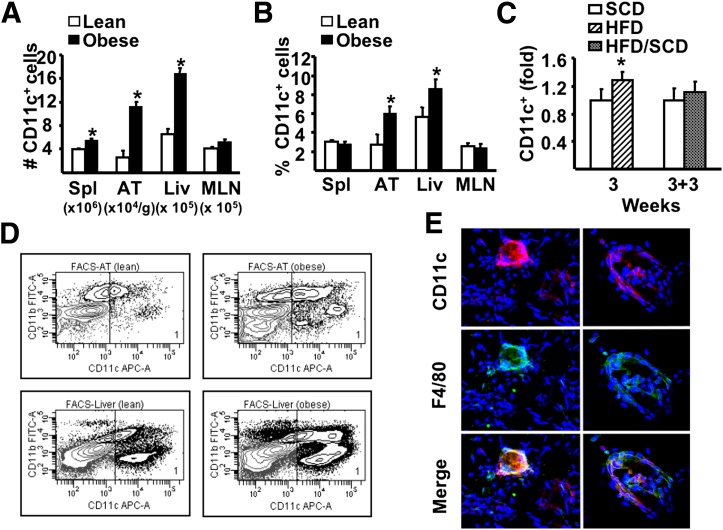
FIG. 2.
The influence of HFD on CD11c+ cells. A and B: After 26 weeks of dietary exposure, mononuclear cells were isolated from spleen (Spl), adipose tissue (AT), liver (Liv), and MLN and stained for specific markers, then analyzed by flow cytometry. A: Number of CD11c+ cells in tissues. B: Proportion of CD11c+ cells among total mononuclear cells isolated from spleen, liver, and MLNs and SVC from AT. C: Fold changes in liver CD11c+ cells in 3-week SCD or HFD or 3-week SCD or HFD followed by the SCD for additional 3 weeks (3 + 3 weeks). D: Representative flow cytometry plots of CD11c+ cells (gate 1) isolated from AT and liver of lean and obese animals. For all experiments above, a minimum of 6 animals per group were individually analyzed (n = 6). Results are presented as means ± SE. Significant differences are indicated (*P < 0.05). E: Representative immunofluorescence of epididymal fat pads obtained from lean and obese animals. After fixation in 2% paraformaldehyde, ∼1 mm3 of tissue was labeled in suspension using rat anti-mouse F4/80 (clone 6F12) and hamster anti-mouse CD11c, both at 1:100 dilution (BD Pharminogen). Goat anti-rat Alexa Fluor 488 (1:500; Invitrogen) and goat anti-hamster Cy3 (1:1,000; Jackson ImmunoResearch Laboratories, West Grove, PA) were used as secondary antibodies. Confocal stack tissue reconstructions of 50 µm were taken at 5 µm intervals using an Olympus Fluoview 1000 Microscope (Olympus, Center Valley, PA). FACS, fluorescence-activated cell sorter; FITC, fluorescein isothiocyanate; APC, allophycocyanin. (A high-quality digital representation of this figure is available in the online issue.)