Abstract
Sodium glucose cotransporter 2 (SGLT2) inhibition is a novel and promising treatment for diabetes under late-stage clinical development. It generally is accepted that SGLT2 mediates 90% of renal glucose reabsorption. However, SGLT2 inhibitors in clinical development inhibit only 30–50% of the filtered glucose load. Why are they unable to inhibit 90% of glucose reabsorption in humans? We will try to provide an explanation to this puzzle in this perspective analysis of the unique pharmacokinetic and pharmacodynamic profiles of SGLT2 inhibitors in clinical trials and examine possible mechanisms and molecular properties that may be responsible.
Type 2 diabetes is a serious global health issue that has reached epidemic proportions in both developed and developing countries over the last two decades (1). With currently available medicines, many diabetic patients fail to achieve optimal glycemic control (HbA1c <6.5–7.0%). With the exception of the glucagon-like peptide 1 analogs and the thiazolidinediones (2), other antidiabetic medications lose their effectiveness to control hyperglycemia over time, partially due to the progressive decline of β-cell function (2–4). As a consequence, many patients receive multiple antidiabetic medicines and eventually require insulin therapy, which often fails to achieve the desired glycemic goal and is associated with weight gain and hypoglycemia (5,6). Failure to achieve glycemic targets is the primary factor responsible for the microvascular complications (retinopathy, neuropathy, nephropathy) and, to a lesser extent, macrovascular complications (2,7). In addition, the majority of diabetic patients are overweight or obese, and many of the current therapies are associated with weight gain, which causes insulin resistance and deterioration in glycemic control (2).
Given the difficulty in achieving optimal glycemic control (8,9) for many diabetic patients using current therapies, there is an unmet medical need for new antidiabetic agents. Although it has been known for 50 years (10,11) that renal glucose reabsorption is increased in type 2 diabetic patients, only recently have the clinical therapeutic implications of this observation been recognized (2,12). Inhibition of renal tubular glucose reabsorption, leading to a reduction in blood glucose concentration through enhanced urinary glucose excretion, provides a novel insulin-independent therapy (2,12) that in animal models of diabetes has been shown to reverse glucotoxicity and improve insulin sensitivity and β-cell function (13,14). The majority (∼80–90%) of filtered plasma glucose is reabsorbed in the early proximal tubule by the high-capacity, low-affinity sodium glucose cotransporter (SGLT) 2 (15,16). The remaining 10–20% of filtered glucose is reabsorbed by the high-affinity, low-capacity SGLT1 transporter in the more distal portion of the proximal tubule. After glucose is actively reabsorbed by SGLT2 and SGLT1 into the proximal tubular cells, it is diffused out of the cells from the basolateral side into blood through facilitative GLUT 2 and 1 (15). Because the majority of glucose reabsorption occurs via the SGLT2 transporter, pharmaceutical companies have focused on the development of SGLT2 inhibitors, and multiple SGLT2 inhibitors currently are in human phase II and III clinical trials (17). This class of antidiabetic medication effectively lowers blood glucose levels and offers additional benefits, including weight loss, low propensity for causing hypoglycemia, and reduction in blood pressure. The SGLT2 inhibitors are effective as monotherapy and in combination with existing therapies (2,12,14,15,17), including insulin (18). Because of their unique mechanism of action (12,15), which is independent of the severity of insulin resistance and β-cell failure, type 2 diabetic individuals with recent-onset diabetes (<1 year) respond equally well as type 2 diabetic patients with long-standing diabetes (>10 years) (19).
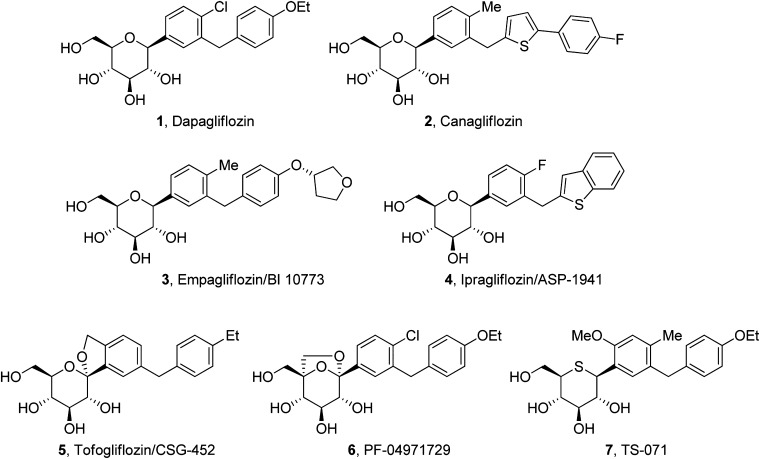
Dapagliflozin is the most advanced SGLT2 inhibitor in clinical trials (12,17,20). In addition, multiple other SGLT2 inhibitors are in phase II to III trials (Fig. 1) (17,21). However, none of these SGLT2 inhibitors are able to inhibit >30–50% of the filtered glucose load, despite in vitro studies indicate that 100% inhibition of the SGLT2 transporter should be achieved at the drug concentrations in humans (22,23). In this perspective, we shall examine potential explanations for this apparent paradox. Resolution of the paradox has important clinical implications with regard to the efficacy of this class of drugs and the development of more efficacious SGLT2 inhibitors.
FIG. 1.
SGLT2 inhibitors in late-stage clinical trials.
PUZZLE ABOUT SGLT2 INHIBITORS
In healthy nondiabetic humans, ∼160–180 g of plasma glucose is filtered daily (glomerular filtration rate [GFR] = 180 L/day × plasma glucose = 900–1000 mg/L), and essentially all of the filtered glucose is reabsorbed in the proximal tubule of the kidneys. It is generally believed that SGLT2 reabsorbs 80–90% of the filtered glucose load (15,16). However, SGLT2 inhibitors in clinical development induce a maximum of 50–80 g of urinary glucose excretion (UGE) per day (i.e., only 30–50% of the filtered glucose load) in healthy volunteers. Some SGLT2 inhibitors cause a maximum daily UGE at a low dose and cannot augment UGE even with a >10-fold increase in dose (22,23). For example, dapagliflozin produces a maximum UGE of ∼60 g/day at a dose of 20 mg/day in healthy human volunteers, and UGE remains at 60 g/day when the dose is increased to 500 mg/day (23). Why can these inhibitors not block 90% of the filtered glucose load in humans?
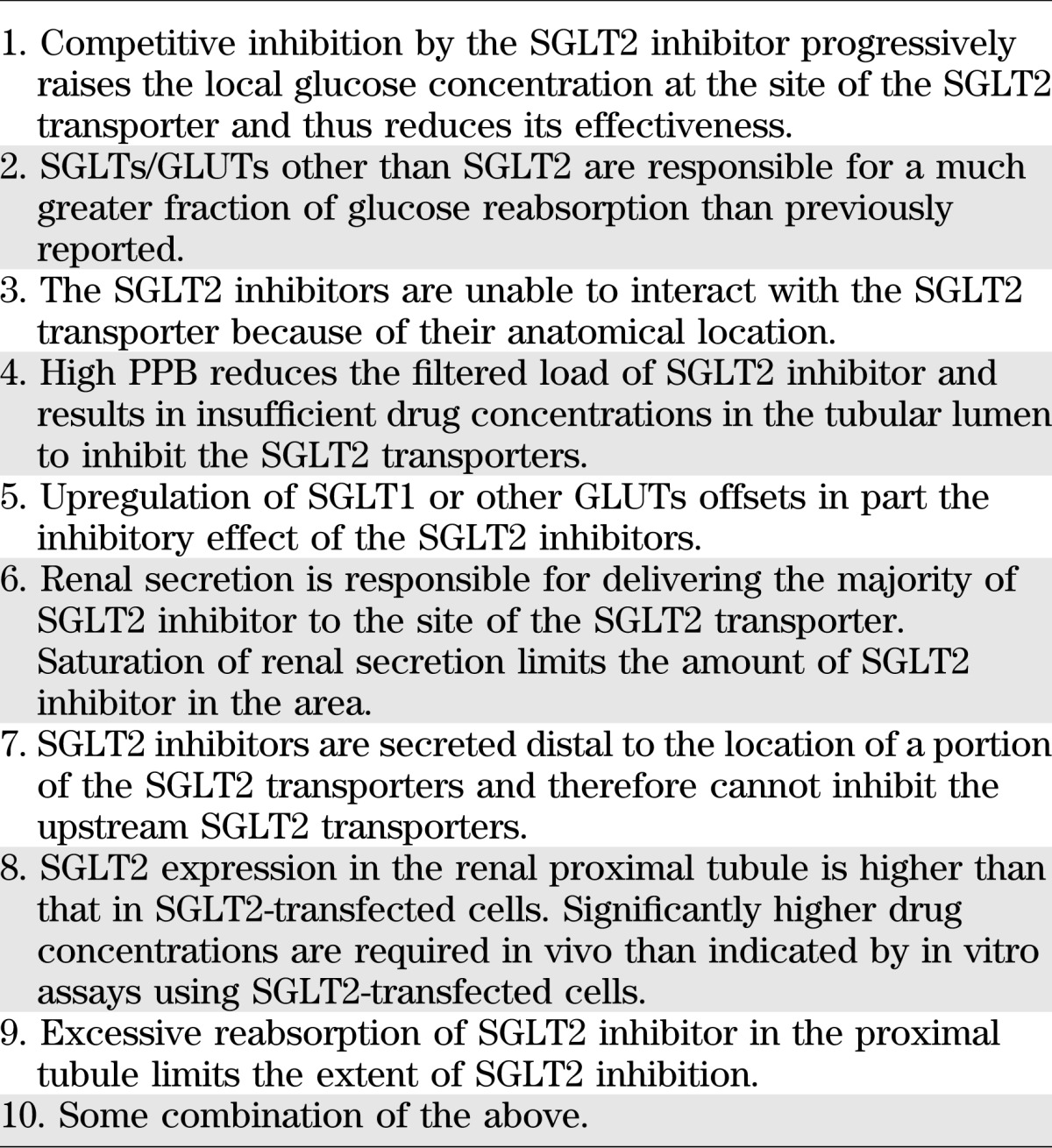
A number of explanations have been proposed to explain this paradox (Table 1, explanations 1–5), but they are insufficient to account for many of the data and observations. In this perspective, we will analyze these explanations and attempt to provide a more satisfactory solution to this puzzle. We will start by examining the pharmacokinetic and pharmacodynamic (PK/PD) characteristics of SGLT2 inhibitors, which are varied and very unique.
TABLE 1.
Potential explanations for why SGLT2 inhibitors cannot inhibit >30–50% of the filtered glucose load
PK/PD CHARACTERISTICS OF SGLT2 INHIBITORS
First, in healthy human volunteers, there are large differences in the dose-dependent PD responses between empagliflozin (BI 10773) and ipragliflozin (ASP-1941) (Fig. 1), despite sharing many similarities (22,24). Both SGLT2 inhibitors have similar chemical structures, human PK profiles, and in vitro potencies. The in vitro SGLT2 half-maximal inhibitory concentration (IC50) of empagliflozin and ipragliflozin is 3.1 and 7.4 nmol/L, respectively. The half-lives (t1/2) of empagliflozin and ipragliflozin in the blood are 8.6–13 and 10–14 h, respectively, and the time to maximum plasma concentration (tmax) is 1.5–2.5 and 1.0–2.3 h, respectively. The area under the curve (AUC) of empagliflozin at the 2.5-mg dose is 396 nmol·h/L and that of ipragliflozin at the 5-mg dose is 810 nmol·h/L. However, UGE over 24 h of empagliflozin at 2.5-mg dose is 30.6 g, whereas that of ipragliflozin at 5-mg dose is only 3.1 g. Differences in plasma protein binding (PPB) can result in different PD responses, but it is likely (although not measured) that the PPB of the two compounds is similar, because the time course PK profiles of the two compounds (as characterized by their t1/2, tmax, and AUC) are almost identical. A difference in PPB would be expected to change the time course PK profile, because this would change the clearance and volume of distribution of the drugs and thus the t1/2. Further, there are no reports in the literature that describe a major difference in PPB of the SGLT2 inhibitors in current clinical development.
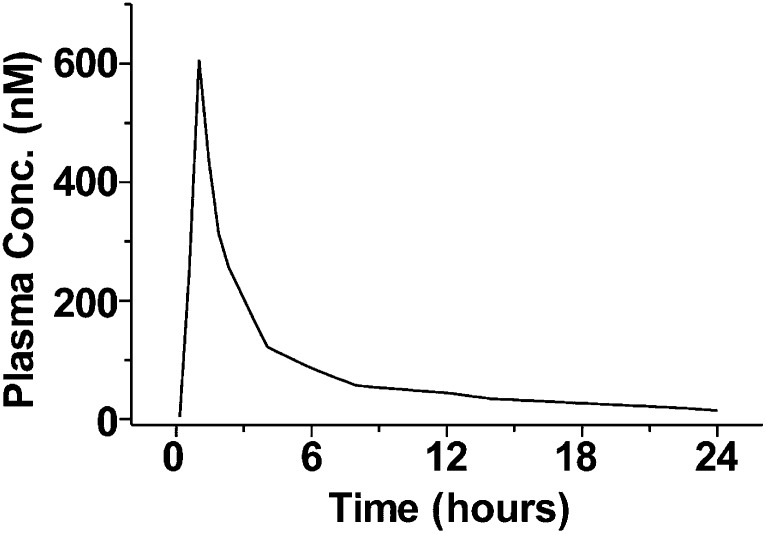
Second, the unbound plasma concentrations of dapagliflozin do not seem to account for the drug’s effect on UGE. In phase I single and multiple ascending-dose studies of dapagliflozin, a near-maximal UGE was maintained in healthy volunteers for 24 h following a dose of 20 mg, whereas the plasma concentration decreased to a range of 10–20 nmol/L at 24 h from a maximum concentration (Cmax) of 600–700 nmol/L (Fig. 2) (21). Taking into account PPB, this corresponds to an unbound dapagliflozin plasma concentration of <2 nmol/L 24 h after the dose. This concentration is in the vicinity of the drug’s in vitro SGLT2 IC50 (1.1 nmol/L), yet dapagliflozin is still capable of generating a near maximum UGE response. To our knowledge, there are no published data that suggest that in vitro potency overestimates the in vivo drug concentration needed to inhibit SGLT2. To the contrary, much higher concentrations of T-1095A (an O-glucoside inhibitor) were required to inhibit glucose uptake into brush border membrane vesicles prepared from animal kidneys than into cells overexpressing SGLT2 (25). It is possible the SGLT2 expression levels in the renal proximal tubule are higher than those on SGLT2-transfected cells. This possibility is supported by studies that have measured the binding of tritium-labeled dapagliflozin to the same amount of protein from mouse kidneys or transfected cells (Amgen, unpublished data). It was found that SGLT2 expression (calculated from the amount of radioactivity bound) in the mouse kidney tissue was several fold higher than in the proteins of transfected cells (Supplementary Fig. 1; T.L., unpublished observations). Because proximal tubular proteins only comprise a small portion of the kidney tissue, the SGLT2 expression levels in the proximal tubule could be substantially higher than those of SGLT2-transfected cells. Considering dapagliflozin’s high selectivity and specificity for SGLT2, nonspecific binding is unlikely to be a significant contributing factor.
FIG. 2.
Plasma dapagliflozin concentration as a function of time following an oral dose of 20 mg in healthy volunteers. Conc., concentration. Modified from Fig. 4 of Komoroski et al. (23).
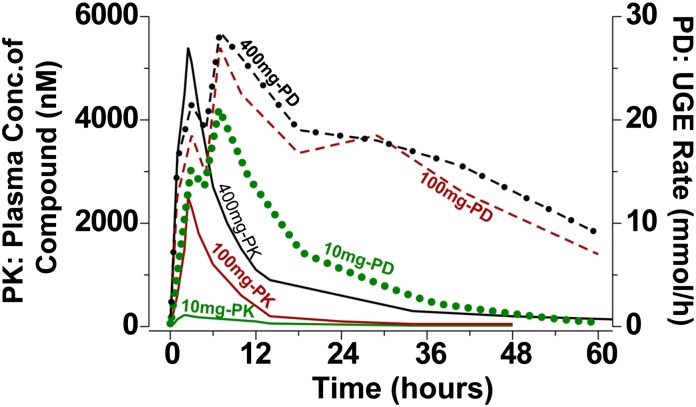
Third, there are disconnects between the UGE effect of empagliflozin and its plasma concentration. After administration of single doses of empagliflozin to healthy humans, a rapid onset of UGE response occurred and was maintained long after the plasma concentrations diminished to very low levels (Fig. 3) (22,26). The empagliflozin plasma concentrations peaked ∼2 h postdose, whereas the maximal UGE rate occurred at about 7 h across a range of doses (10 to 800 mg). Further, the UGE rates did not decline nearly as rapidly as the decrease in plasma concentrations. Following the 100- and 400-mg doses of empagliflozin, the UGE rates at 60 h postdose dropped about threefold from the peak values, whereas the plasma concentrations at the same doses dropped >50-fold from their peak levels during the same period of time.
FIG. 3.
Schematic PK/PD graphs of empagliflozin (BI 10773). UGE rate–time profiles are shown in dotted line (10 mg-PD), dashed line (100 mg-PD), and dashed-dotted line (400 mg-PD). Plasma concentration–time profiles are shown in solid lines. Three representative doses are shown: 10, 100, and 400 mg. Conc., concentration. Modified from Port et al. (22) and Dugi and Mark (26).
The unbound plasma drug concentration usually correlates well with PD response. In the case of SGLT2 inhibitors, it also appears reasonable to assume that the unbound plasma concentration reflects the concentration in the location of the SGLT2 transporters. Unbound SGLT2 inhibitors are filtered through glomeruli and travel a short distance to reach the SGLT2 transporters, which are located in the early segment of the renal proximal tubule. However, the three observations above indicate that the UGE responses induced by the SGLT2 inhibitors do not correlate with the drug’s unbound plasma concentrations. Why? In the following discussion, we provide a possible answer to this question, followed by an explanation for the puzzle described in the title.
EXPLANATION OF THE PARADOX
As described above, SGLT2 inhibitors, such as dapagliflozin and empagliflozin, demonstrate unique PK/PD relationships. Robust UGE was maintained long after the plasma drug concentrations diminished (22,23) (Fig. 3). If SGLT2 inhibitors are actively secreted (in addition to filtered) into the renal proximal tubule, the renal secretion can deliver higher drug concentrations to the area of the SGLT2 transporter and thus contribute to the robust UGE effect, especially when the plasma concentrations are low. Another possible contributing factor that may explain the prolonged UGE effect is a slow off rate of SGLT2 inhibitors. Compounds with a slow off rate can maintain their PD effect even when their plasma concentrations are low. Empagliflozin has a slow off rate with a dissociation t1/2 of 1 h both in the absence and presence of 20 mmol/L glucose (27). If SGLT2 inhibitors also block the transporter from inside the cells, their inhibition effect can last longer than indicated by their dissociation t1/2 (28).
To the best of our knowledge, renal secretion has not been considered as a factor for the PK/PD relationship in the literature. There are several potential reasons for the lack of consideration of renal secretion. First, the reported renal clearance of SGLT2 inhibitors is low across species and insignificant compared with their hepatic clearance (29,30). Dapagliflozin has a low renal clearance of 5.6 mL/min in humans, half of its GFR*fu (10.8 mL/min), in which fu is fraction unbound (29). When renal clearance is insignificant and less than the product of GFR*fu, renal secretion is not considered to be a significant factor for drug–drug interaction (DDI), so it is not critical to study renal secretion and the transporters involved for compounds with low renal clearances (31). Second, even if renal transporters are studied, the ones most often examined for DDI are limited, such as P-glycoprotein, breast cancer–resistant protein, organic anion transporters, and organic cation transporters (OCTs) (31). There are many more transporters (peptide transporters 1 and 2, multidrug resistance–associated protein 2 and 4, OCTN1, and OCTN2, etc.) involved in renal secretion, but often they are not studied for DDI (31).
Low renal clearance does not mean lack of renal secretion, because significant renal secretion can be masked by renal reabsorption, if compounds have good passive permeability (32–34). SGLT2 inhibitors generally have good permeability, as indicated by high oral bioavailability and permeability measurement (29). Although there is no direct evidence in the literature to support renal secretion of SGLT2 inhibitors, indirect evidence supports such a mechanism. It is interesting to note that following oral administration of a 1 mg/kg dose of TS-071 (Fig. 1), rats exhibited kidney/plasma ratios of 35 at 4 h postdose, despite the fact that TS-071 primarily is excreted by hepatic metabolism (35). After 24 h, TS-071 was no longer detected in rat kidneys. Renal secretion and slow off rate from targets in the kidney can make compounds preferentially distributed into the kidney.
Neutral drugs that have high lipophilicity (indicating good permeability), like dapagliflozin, tend to have a low renal clearance (33). Even though the renal clearance of dapagliflozin is low, many drugs that have similar PPB (indicated by GFR*fu) and similar lipophilicity (indicated by cLogD or cLogP) to those of dapagliflozin actually have a much lower renal clearance than dapagliflozin (34). The reason why dapagliflozin does not have a lower renal clearance may be due to its renal secretion.
The sustained robust UGE and different timing of peak UGE and Cmax of empagliflozin also suggest renal secretion (Fig. 3) (22,26). If glomerular filtration were the only way for SGLT2 inhibitors to reach the site of action, one would expect the timing of peak UGE to match the tmax of the plasma concentration and UGE to decrease in concert with the reduction of the plasma concentration. The onset of UGE response is rapid after SGLT2 inhibition. Although slow off rate of SGLT2 inhibitors can contribute to the slow decrease of UGE response, it cannot explain the vastly slower decline of UGE response compared with the plasma concentration.
The human metabolite profile of dapagliflozin suggests that active metabolites do not significantly contribute to the PD response, because dapagliflozin is primarily eliminated as a pharmacologically inactive glucuronide metabolite (29). The possibility of superpotent minor metabolites contributing to the PD effects cannot be ruled out, but there is no evidence to support this.
If SGLT2 inhibitors are secreted by the renal tubule, this could provide a solution to the puzzle as to why SGLT2 inhibitors are unable to inhibit 80–90% of the filtered glucose load in humans. First, even though renal secretion may deliver sufficient drug to achieve robust UGE for a longer duration, it can saturate at high doses and thus limit SGLT2 inhibitors from maximally inhibiting renal glucose reabsorption (Table 1, explanation 6). Second, location of renal secretion relative to that of the SGLT2 transporters can affect the efficiency of SGLT2 inhibition. Downstream renal secretion of drug cannot affect glucose reabsorption, if the SGLT2 transporters are located upstream (Table 1, explanation 7). Third, full inhibition of the SGLT2 transporter may require significantly higher concentrations of the inhibitors than suggested by the in vitro SGLT2 potency, because the expression levels of SGLT2 in the renal proximal tubule may be much higher than those on the transfected cells used in the in vitro assays (Table 1, explanation 8). Fourth, SGLT2 inhibitors delivered through glomerular filtration may have an impact on UGE during the initial few hours after drug administration, but exert limited effect at later times even at high doses, because the plasma concentration declines rapidly after the Cmax is reached. Taken collectively, it is possible that glomerular filtration cannot deliver impactful levels of the SGLT2 inhibitors over a long duration even when high doses of the drugs are given. Rather, renal tubular secretion and slow off rate of the inhibitors may be responsible for the sustained drug levels in the proximal tubule, which in turn results in a sustained UGE response. Full inhibition of SGLT2 may be limited by the capacity of renal secretion and/or the proximity of the site of renal secretion and the SGLT2 transporters.
It also is possible that excessive reabsorption of SGLT2 inhibitors in the proximal tubule contributes to the inability of SGLT2 inhibitors to block >30–50% of glucose reabsorption (Table 1, explanation 9). Excessive active reabsorption through transporters could result in significantly lower drug concentrations in the proximal tubule than the plasma unbound concentration and thus limit the inhibition. But the lower tubular concentration is not consistent with the prolonged robust UGE effect, unless inhibition of the SGLT2 transporter also can take place intracellularly. Active reabsorption can accumulate SGLT2 inhibitors inside the tubular cells and result in the prolonged UGE effect (especially in combination with slow off rate), if the inhibitors also block from inside the cells. In this case, the capacity of the active reabsorption and/or its location relative to the SGLT2 transporters dictates the extent of SGLT2 inhibition. However, there is no evidence in the literature regarding possible intracellular inhibition of the SGLT2 transporter by the SGLT2 inhibitors in clinical trials. Passive reabsorption does not have the same effect as active reabsorption, because it is driven by the drug concentration gradient from the renal tubule to the bloodstream. Therefore, it only makes the drug tubular concentration approach the plasma unbound concentration.
Renal secretion (and/or reabsorption) also can explain several other important observations. First, as discussed above, empagliflozin and ipragliflozin have very different dose-dependent UGE responses, even though they share many similar properties, such as potency and PK properties. It is possible that differences in renal secretion (and/or reabsorption) and dissociation t1/2 are responsible for the different UGE responses. Second, in contrast to humans, SGLT2 inhibitors can block >70% of the glucose reabsorption in Sprague-Dawley rats. In these rats, about 3.6 g/day/200 g body weight of glucose is filtered through glomeruli (GFR = 1.4 mL/min/100 g body weight = 4 L/day/200 g body weight × 0.9 g/L = plasma glucose level) (36). The maximum UGE/day/200 g body weight induced by SGLT2 inhibitors in Sprague-Dawley rats is 2.6 g for dapagliflozin and PF-04971729 (37,38), 2.9 g for EGT1442 (39), and 3.7 g for canagliflozin (40). Potency and PK cannot explain this species difference. This difference may be explained by a greater renal secretory (and/or reabsorption) capacity and/or more upstream renal secretion (and/or reabsorption) in rodents compared with humans.
There are other potential explanations (Table 1, explanations 1–5) for this puzzle, but they are unlikely to explain the paradox. The first explanation in Table 1 is that increased levels of glucose in the proximal tubule, as a result of SGLT2 inhibition, compete with the drug and thus prevent the full inhibition of SGLT2. However, this is not consistent with the observation that a >20-fold increase in the dose of dapagliflozin from 20 to 500 mg/day only prolongs the PD effect and does not further increase the level of inhibition (∼40% inhibition of the glucose reabsorption) (23). The second explanation in Table 1 is that GLUT other than SGLT2 may be responsible for a much greater fraction of the glucose reabsorption. However, this cannot explain why some patients with severe SGLT2 mutations excrete >150 g/day of glucose into the urine, unless the SGLT2 mutation also affects other glucose transporters (i.e., SGLT5 or SGLT1, for which genes are not located on the same chromosome as SGLT2). In addition, LX-4211, a SGLT2/SGLT1 dual inhibitor in phase II clinical trials, does not generate a larger glucosuric effect than selective SGLT2 inhibitors (41). SGLT5 has been suggested as a transporter that may be responsible for a greater fraction of glucose reabsorption, because of its almost exclusive expression in the renal tubule and its unidentified function. However, recent studies have demonstrated that SGLT5 is a mannose fructose transporter (27). Further, remogliflozin is a SGLT2/SGLT5 dual inhibitor with IC50 for the two transporters of 12 and 480 nmol/L, respectively (42). In healthy humans, remogliflozin etabonate (prodrug of remogliflozin) could not induce more than ∼40 g/day of UGE, even when its dose was increased from 150 to 1000 mg (43). Based upon these observations, the role of SGLT5 in renal glucose reabsorption appears to be insignificant. Furthermore, knockout of SGLT2 in the mouse results in the excretion of 70–80% of the filtered glucose load (16), although one could argue that other GLUTs play a more dominant role in man. Last and most important, SGLT2 antisense oligonucleotides, which knock down SGLT2 mRNA expression up to 80%, have a much greater glucosuric effect than the orally administered SGLT2 inhibitors in multiple species, including rats and dogs as well as monkeys (44,45).
Explanations 3–5 in Table 1 also are unlikely. Based upon animal studies that have localized the SGLT2 transporter to the brush border membrane of the proximal tubule (16), inability of the filtered SGLT2 inhibitor to interact with the SGLT2 transporters because of their anatomical location seems unlikely (Table 1, explanation 3). The high PPB of some SGLT2 inhibitors should not limit the extent of inhibition if the dose is increased (Table 1, explanation 4). High PPB limits the percentage of unbound drug and thus limits the amount of drug filtered through the glomeruli. However, the amount of filtered (unbound) drug can be increased, if the dose is increased. For example, unbound Cmax of dapagliflozin increases from ∼60 nmol/L to >1 μmol/L (calculated using 91% PPB and its PK in healthy humans) (23,29) when its dose is increased from 20 to 500 mg. The similar magnitude of glucosuria on days 1–3 versus day 14 after administration of dapagliflozin strongly argues against upregulation of other SGLTs or other GLUTs distal to the SGLT2 transporter (Table 1, explanation 5) (46). Moreover, phlorizin-treated diabetic rats do not upregulate GLUT2 (47). Although SGLT2 inhibition does not appear to cause upregulation of SGLTs or other GLUTs in humans, there is evidence for increased GLUT2 expression in diabetic rodent models (17,19). However, in healthy humans in whom upregulation of GLUTs and/or SGLTs does not occur, SGLT2 inhibitors still cannot block >30–50% of renal glucose reabsorption. In addition, if there were significant contribution to glucose reabsorption from GLUTs or SGLTs other than SGLT2, it would not explain the unique PD effects of SGLT2 inhibitors, such as prolonged maximum UGE response at diminished plasma levels and different timing between the maximum PD response of empagliflozin and its maximum plasma concentration.
CONCLUSIONS
The SGLT2 inhibitors in human clinical trials have good efficacy, but they do not inhibit >30–50% of the filtered glucose load. Based upon their PK/PD relationship, we postulate that their mechanism of action is related to secretion and/or active reabsorption in the proximal tubule and slow off rate from the SGLT2 target. Renal micropuncture studies with radiolabeled SGLT2 inhibitors and comparison of drug radioactivity in the glomerular filtrate and proximal tubule will help to define the relative contributions of tubular secretion and/or reabsorption versus glomerular filtration in establishing the drug concentration at the site of the SGLT2 transporters in the proximal tubule. A better understanding of the renal handling of the SGLT2 inhibitors will help to develop more effective medications that are capable of inhibiting a greater percentage of filtered glucose load and causing a greater reduction in HbA1c.
ACKNOWLEDGMENTS
R.A.D. received grants from Amylin and Takeda; served on the advisory boards of Amylin, Takeda, BMS, Novo Nordisk, Jansen, and Boehringer-Ingelheim; and served as a member of the Speakers Bureau at Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
J.L. and R.A.D. wrote, reviewed, and edited the manuscript. T.L. researched data and reviewed and edited the manuscript. J.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Ernest M. Wright of David Geffen School of Medicine at University of California Los Angeles and Dr. Jeff Jasper of Cytokinetics for editorial comments.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0052/-/DC1.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2011. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011 [Internet]. Atlanta, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available from www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed 11 February 2011
- 2.DeFronzo RA. Banting lecture. From the triumvirate to the omnious octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853, 853–865 [PubMed]
- 4.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010;33:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry RR, Gumbiner B, Ditzler T, Wallace P, Lyon R, Glauber HS. Intensive conventional insulin therapy for type II diabetes: metabolic effects during a 6-month outpatient trial. Diabetes Care 1993;16:21–31 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AWK, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009;122:443–453 [DOI] [PubMed] [Google Scholar]
- 9.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 10.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 1971;28:101–109 [DOI] [PubMed] [Google Scholar]
- 11.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 1951;30:125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515–531 [DOI] [PubMed] [Google Scholar]
- 13.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733–794 [DOI] [PubMed] [Google Scholar]
- 16.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011;22:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5–14 [DOI] [PubMed] [Google Scholar]
- 18.Wilding JP, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 2009;32:1656–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab 2010;12:510–516 [DOI] [PubMed] [Google Scholar]
- 20.Jones D. Diabetes field cautiously upbeat despite possible setback for leading SGLT2 inhibitor. Nat Rev Drug Discov 2011;10:645–646 [DOI] [PubMed] [Google Scholar]
- 21.Kipnes MS. Sodium–glucose cotransporter 2 inhibitors in the treatment of Type 2 diabetes: a review of Phase II and III trials. Clin Invest 2011;1:145–156 [Google Scholar]
- 22.Port A, Macha S, Seman L, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of BI 10773, a sodium glucose cotransporter-2 (SGLT2) inhibitor, in healthy volunteers. Presented at the 70th Annual Meeting of the American Diabetes Association, 25–29 June 2010, Orlando, Florida [Google Scholar]
- 23.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009;85:520–526 [DOI] [PubMed] [Google Scholar]
- 24.Veltkamp SA, Kadokura T, Krauwinkel WJJ, Smulders RA. Effect of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig 2011;31:839–851 [DOI] [PubMed] [Google Scholar]
- 25.Oku A, Ueta K, Arakawa K, et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 1999;48:1794–1800 [DOI] [PubMed] [Google Scholar]
- 26.Dugi K, Mark M. Boehringer-Ingelheim R&D press conference [Internet], 17 October 2008. Available from www.boehringer-ingelheim.com/content/.../slides_mark_dugi.pdf Accessed 16 April 2011
- 27.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012;14:83–90 [DOI] [PubMed] [Google Scholar]
- 28.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 2006;5:730–739 [DOI] [PubMed] [Google Scholar]
- 29.Obermeier M, Yao M, Khanna A, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos 2010;38:405–414 [DOI] [PubMed] [Google Scholar]
- 30.Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF-04971729, a selective inhibitor of the sodium-dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos 2011;39:1609–1619 [DOI] [PubMed] [Google Scholar]
- 31.Giacomini KM, Huang SM, Tweedie DJ, et al. International Transporter Consortium Membrane transporters in drug development. Nat Rev Drug Discov 2010;9:215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng B, LaPerle JL, Chang G, Varma MV. Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol 2010;6:939–952 [DOI] [PubMed] [Google Scholar]
- 33.Li M, Anderson GD, Wang J. Drug-drug interactions involving membrane transporters in the human kidney. Expert Opin Drug Metab Toxicol 2006;2:505–532 [DOI] [PubMed] [Google Scholar]
- 34.Varma MV, Feng B, Obach RS, et al. Physicochemical determinants of human renal clearance. J Med Chem 2009;52:4844–4852 [DOI] [PubMed] [Google Scholar]
- 35.Kakinuma H, Oi T, Hashimoto-Tsuchiya Y, et al. (1S)-1,5-anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a potent, selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes treatment. J Med Chem 2010;53:3247–3261 [DOI] [PubMed] [Google Scholar]
- 36.Schock-Kusch D, Sadick M, Henninger N, et al. Transcutaneous measurement of glomerular filtration rate using FITC-sinistrin in rats. Nephrol Dial Transplant 2009;24:2997–3001 [DOI] [PubMed] [Google Scholar]
- 37.Robinson RP, Mascitti V, Boustany-Kari CM, et al. C-Aryl glycoside inhibitors of SGLT2: Exploration of sugar modifications including C-5 spirocyclization. Bioorg Med Chem Lett 2010;20:1569–1572 [DOI] [PubMed] [Google Scholar]
- 38.Mascitti V, Maurer TS, Robinson RP, et al. Discovery of a clinical candidate from the structurally unique dioxa-bicyclo[3.2.1]octane class of sodium-dependent glucose cotransporter 2 inhibitors. J Med Chem 2011;54:2952–2960 [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Welihinda A, Mechanic J, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res 2011;63:284–293 [DOI] [PubMed] [Google Scholar]
- 40.Nomura S, Sakamaki S, Hongu M, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem 2010;53:6355–6360 [DOI] [PubMed] [Google Scholar]
- 41.Powell D. Oral presentation OR24-6 [article online]. Presented at the Endocrine Society Annual Meeting, 19–22 June 2010, San Diego, California. Available from http://www.lexicon-genetics.com/~lexpha5/images/pdfs/LX4211_ENDO2010_Presentation.pdf Accessed 21 December 2011
- 42.Grempler R, Thomas L, Eckhardt M, et al. In Vitro Properties and In Vivo Effect on Urinary Glucose Excretion of BI 10773, a Novel Selective SGLT2 Inhibitor. Presented at the 69th Annual Meeting of the American Diabetes Association, 5–9 June 2009, New Orleans, Louisiana [Google Scholar]
- 43.Kapur A, O’Connor-Semmes RL, Hussey EK, et al. First Human Dose Escalation Study with Remogliflozin Etabonate (RE) in Healthy Subjects and in Subjects with Type 2 Diabetes Mellitus (T2DM). Presented at the 69th Annual Meeting of the American Diabetes Association, 5–9 June 2009, New Orleans, Louisiana [Google Scholar]
- 44.Wancewicz E, Siwkowski A, Meibohm B, et al. Long term safety and efficacy of ISIS 388626, An optimized SGLT2 antisense inhibitor, in multiple diabetic and euglycemic species. Presented at the 68th Annual Meeting of the American Diabetes Association, 6–10 June 2008, San Francisco, California [Google Scholar]
- 45.Bhanot S, Murray SF, Booten SL, et al. ISIS 388626, an SGLT2 antisense drug, causes robust and sustained glucosuria in multiple species and is safe and well-tolerated (Abstract). Diabetes 2009;58(Suppl. 1):A328 [Google Scholar]
- 46.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freitas HS, D’Agord Schaan B, da Silva RS, Okamoto MM, Oliveira-Souza M, Machado UF. Insulin but not phlorizin treatment induces a transient increase in GLUT2 gene expression in the kidney of diabetic rats. Nephron Physiol 2007;105:p42–p51 [DOI] [PubMed] [Google Scholar]