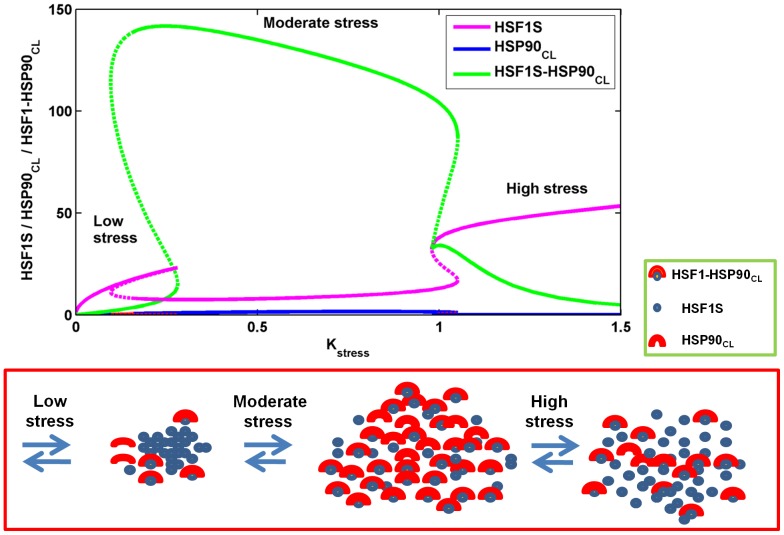
Figure 9. Dynamics of stress-initiated molecular events in heat-shock protein 90 (HSP90) production.
Three different levels of stress, low, moderate, and high, are considered in mapping the molecular events that drives the mushroom dynamics of the HSP90 network using the bifurcation diagram. An illustration of the molecular events corresponding to the occurrence of mushroom dynamics in terms of heat-shock factor 1 monomer (HSF1S, blue filled circle), the closed conformation of heat-shock protein 90 (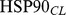 , in yellow-filled red semicircle) and
, in yellow-filled red semicircle) and 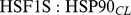 complex (in yellow-filled red semi-circle bound to blue filled circle) are shown in the lower panel. A low level of stress increases the inactive HSF1, and converts it rapidly into an active form, HSF1S.
complex (in yellow-filled red semi-circle bound to blue filled circle) are shown in the lower panel. A low level of stress increases the inactive HSF1, and converts it rapidly into an active form, HSF1S. 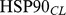 is present at a low level due to the time taken for its production. An increase in stress to a moderate level results in further production of active HSF1S. This in turn oligomerizes, and through a sequence of PdP reactions, produces more
is present at a low level due to the time taken for its production. An increase in stress to a moderate level results in further production of active HSF1S. This in turn oligomerizes, and through a sequence of PdP reactions, produces more 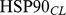 . This negatively regulates its own production by rapidly forming a complex with the inactive HSF1 monomer, and results in a drastic reduction of its own concentration. For a very high stress level,
. This negatively regulates its own production by rapidly forming a complex with the inactive HSF1 monomer, and results in a drastic reduction of its own concentration. For a very high stress level, 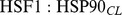 complex breaks down rapidly, and the inactive HSF1 is again converted into a more active form, HSF1S.
complex breaks down rapidly, and the inactive HSF1 is again converted into a more active form, HSF1S.