Abstract
Background
Impact injury to articular cartilage can lead to posttraumatic osteoarthritis.
Hypotheses
This study tests the hypotheses that (1) chondrocyte injury occurs after impact at energies insufficient to fracture the cartilage surface, and that (2) cartilage injury patterns vary with impact energy, time after injury, and cartilage thickness.
Study Design
Controlled laboratory study.
Methods
Fresh bovine osteochondral cores were randomly divided into 5 groups: (1) control, (2) 0.35 J, (3) 0.71 J, (4) 1.07 J, and (5) 1.43 J impact energies. Cores were subjected to computer-controlled impact loading and full-thickness sections were then prepared and incubated in Dulbecco's Modified Eagle's Medium/F12 at 37°C. Adjacent sections were harvested 1 and 4 days after impact for viability staining and fluorescent imaging. The area of dead and living chondrocytes was quantified using custom image analysis software and reported as a percentage of total cartilage area.
Results
The highest impact energy fractured the cartilage in all cores (1.43 J, n = 17). Seventy-three percent and 64% of the osteochondral cores remained intact after lower energy impacts of 0.71 J and 1.07 J, respectively. At lower energy levels, fractured cores were thinner (P < .01) than those remaining intact. In cores remaining intact after impact injury, chondrocyte death increased with increasing impact energy (P < .05) and with greater time after impact (P < .05). A progressive increase in dead cells near the bone/cartilage interface and at the articular surface was observed.
Conclusion
These data showing progressive chondrocyte death after impact injury at energies insufficient to fracture the cartilage surface demonstrate a potential need for early chondroprotective therapy.
Clinical Relevance
These data show that efforts to reduce chondrocyte morbidity after joint injury may be a useful strategy to delay or prevent the onset of posttraumatic osteoarthritis.
Keywords: impact injury, articular cartilage, chondrocyte necrosis, posttraumatic osteoarthritis
Articular cartilage is a highly specialized connective tissue that covers the articulating surfaces of synovial joints. Injury to articular cartilage sustained in trauma or during sports activities has been shown to contribute to posttraumatic degenerative osteoarthritis.10 This ailment is indistinguishable in its end stage from primary osteoarthritis and is characterized by progressive cartilage loss, pain, swelling, inflammation, and joint immobility.
Approximately 12% of the overall prevalence of symptomatic osteoarthritis, a leading source of disability, is attributable to posttraumatic osteoarthritis, resulting in a financial burden of $3.06 billion annually in the United States.5 Although the causes of secondary osteoarthritis are not fully understood, multiple studies have shown that matrix degradation and chondrocyte death occur after blunt impact to articular cartilage.4,14,20 A recent study demonstrated release of glycosaminoglycans from explants subjected to high and low rates of loading that was significantly higher than that of nonimpact controls.9 Additionally, matrix disintegration with fissuring and surface fracture may occur after single impact to the articular cartilage.14 Finally, even though cartilage injury models differ and are difficult to compare,28 cell death was found in virtually all studies that examined chondrocyte viability after impact to articular cartilage.§
The overall goal of this study was to characterize zones of chondrocyte death after impact injury at energies insufficient to fracture the cartilage surface. Current studies have shown cell death in the superficial zone of articular cartilage after impact,6,16 with increased layer depth as impact energy increases.18 This study was performed to test the hypotheses that (1) chondrocyte injury occurs after impact at energies insufficient to grossly fracture the cartilage surface, and that (2) cartilage injury patterns vary with impact energy, time after injury, and cartilage thickness.
MATERIALS AND METHODS
Tissue Preparation
Eight bovine knees were obtained from a local abattoir within 4 hours of animal sacrifice. Each knee was opened under sterile conditions via a standard parapatellar arthrotomy and the femoral condyles were exposed. An 8.5-mm coring device (Smith & Nephew, Andover, Massachusetts) was used to harvest osteochondral cores from the femur. Explants were immediately transferred to 24-well plates filled with 2 mL/well of Hank's balanced salt solution (HBSS, Gibco, Grand Island, New York). The location of harvested cores was also recorded. Tissue samples were carefully examined and explants with nonuniform cartilage thickness or excessive surface curvature were excluded. A total of 160 osteochondral cores were used. The cartilage thickness of each core was measured with a caliper and the subchondral bone was trimmed to 1 mm.
Cartilage Impact
Cores were randomly divided into 5 experimental groups: (1) control (no impact), (2) low-energy impact (0.35 J), (3) intermediate-energy impact (0.71 J), (4) high-energy impact (1.07 J), and (5) very high-energy impact (1.43 J). A custom-built impact tower was used to impact freshly harvested and intact articular cartilage cores. Cores were impacted in a size-matched holder (8.5 mm) with a flat cylinder 6.15 mm in diameter. A weight of 356 g was used throughout the study and impact energy was controlled by adjusting the drop height. The load-deformation responses were recorded by an oscilloscope and analyzed to determine impact energy, stress rate, and average peak force.
Evaluation of Cell Death
The purpose of the study was to evaluate chondrocyte death in articular cartilage appearing normal to surface inspection after impact injury. Specimens that fractured during impact were not suitable for this purpose and were excluded. Cores that retained intact articular surface after impact were bisected in the midsagittal plane and two 500-μm full-thickness cartilage sections (4 per core) were obtained. Cartilage/bone slices were incubated (5% CO2, 37°C) in 1 mL of chondrocyte growth medium (Dulbecco's Modified Eagle's Medium [DMEM]/F12 with 10% fetal bovine serum and 1% antibiotics) for 1 day or 4 days after impact and analyzed. Cell viability was determined with live/dead fluorescent staining using 5-chloromethylfluorescein diacetate (CMFDA) CellTracker Green (Invitrogen, Eugene, Oregon) for living and propidium iodide (PI, Invitrogen) for dead cells. Each osteochondral slice was incubated (5% CO2, 37°C) with 2 μM of CMFDA and 1 μM of PI for 60 minutes.
After incubation, stained sections were imaged with an epifluorescent stereomicroscope (MVX10 MacroView System, Olympus, Tokyo, Japan) and a confocal microscope (IX81 DSU, Olympus, Center Valley, Pennsylvania). Areas representing predominantly live or dead chondrocytes on full-thickness images were defined using 3-dimensional, volumetric confocal microscopy to analyze red (n = 9) and green (n = 9) regions.7,8,15,31 Quantification of live and dead cells in these regions showed that red regions contained predominantly dead chondrocytes (96% ± 2% dead cells) and green regions contained mostly viable chondrocytes (18% ± 5% dead cells).
Custom image analysis software (Visiopharm, Hørsholm, Denmark) was used to segment the cartilage into superficial, middle, and deep layers. The area representing dead chondrocytes (red zone) and live chondrocytes (green zone) was then measured for each layer. The percentage area occupied by the dead zone was then calculated.
Statistical Analysis
Statistical analysis was performed using 1-way analysis of variance with Bonferroni posttest or the paired t test or Student t test as appropriate. Statistical significance was set at P < .05.
RESULTS
Increased osteochondral injury was observed with higher impact energies and in specimens with thinner cartilage. Impact with the highest energy (1.43 J) fractured the articular cartilage of all cores tested (n = 17), while at lower energies (0.71 J or 1.07 J), 74% (n = 31 of 42) and 64% (n = 31 of 48) of cores remained intact after impact, respectively (Figure 1). At lower energy levels, fractured cores were on average 20.2% thinner than their intact counterparts (P < .01). In thin osteochondral explants (<2 mm cartilage), 64.7% of cores were severely fractured, while in thicker cores (>2 mm cartilage), only 27.2% fractured.
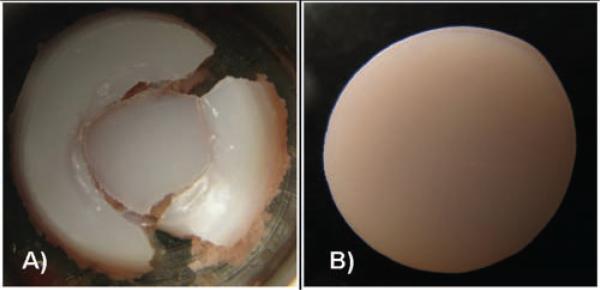
Figure 1.
Representative images of cores subjected to 1.43 J (A) and cores subjected to lower-impact energies (B). All cores subjected to high-energy impact (1.43 J, n = 17) were destroyed. Cores subjected to lower energy impact and controls usually remained intact.
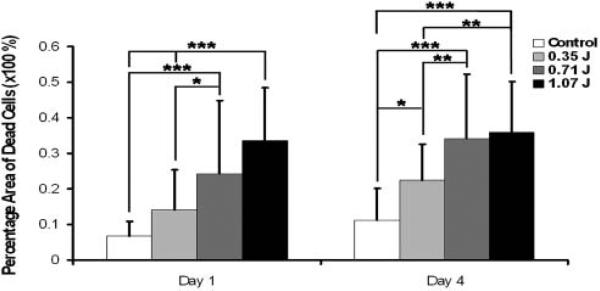
Chondrocyte death increased with higher-impact energies and with greater time after impact in intact cores (Figure 2). Mean cartilage thickness of 2.7 ± 0.7 mm was similar between groups (P = 1.0). One day after impact, an increase to 14.2%, 24.4%, and 33.6% in area of necrotic cells was observed for the low (0.35 J), intermediate (0.71 J), and high (1.07 J) impact energy groups, respectively. The differences were statistically significant when comparing intermediate (0.71 J) and high (1.07 J) impact groups with nonimpacted specimens (P < .001) or with low-impact (0.35 J) samples (0.71 J, P = .033; 1.07 J, P < .001). This phenomenon was also observed on day 4, when the area of dead cells in intermediate (0.71 J) and high (1.07 J) impact groups increased to 34.1% and 35.8% and was larger than nonimpacted controls (P < .001) and low-impact (0.35 J) samples (0.71 J, P = .003; 1.07 J, P = .002) An increase in the area of dead cells between 1 and 4 days after impact was observed for specimens impacted at low (0.35 J, P = .0017) and at medium energy (0.71 J, P = .0079).
Figure 2.
Chondrocyte viability after impact. The percentage area of cell death is greater as impact energy increases and progresses with time. Intermediate- and high-energy impacts have significantly more death than low-energy and nonimpacted samples at both day 1 and day 4. Additionally, at day 4, there is an increase in cell death at low-energy impact when compared with control. Values reported are an average ± standard deviation. (P < .001***, P < .01**, P <.05* vs indicated group.)
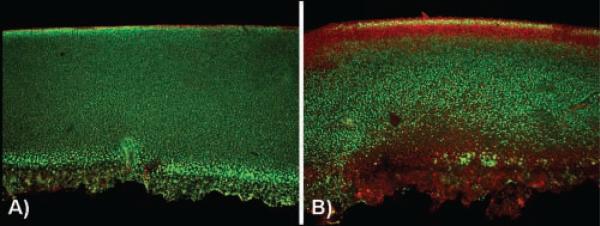
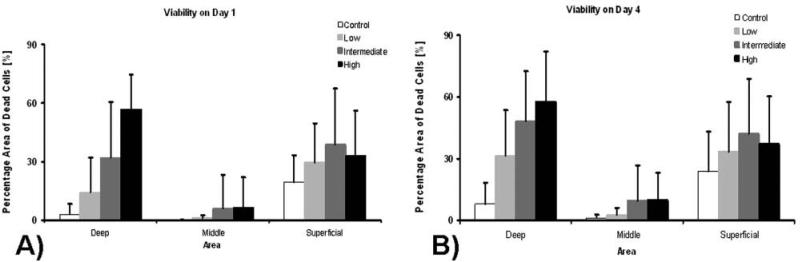
In nonimpacted controls, limited cell death was seen, primarily at the cartilage surface (Figure 3A). In contrast, a consistent pattern of chondrocyte death was seen with low-, moderate-, and high-energy impacts with death localized near the subchondral bone/cartilage interface and at the articular surface (Figure 3). There was a statistically significant decrease in dead cells in the middle region compared with the upper and lower explant regions (P < .01). Further, as impact energy increased, there was a corresponding increase in chondrocyte necrosis, most pronounced near the bone/cartilage interface (Figure 4A). The zone of dead chondrocytes, first localized to the articular surface and bone interface, expands over time, extending into the middle region of the core and resulting in an expanding dead zone as culture time increases (Figure 4B).
Figure 3.
Representative confocal images of core sections stained for living cells (green) and dead cells (red). Nonimpacted control (A), core impacted with energy of 0.71 J (B); a characteristic chondrocyte death pattern is visible at the site of impact (on the surface) and in the deep basal zone of the cartilage (at the subchondral bone/cartilage interface).
Figure 4.
Chondrocyte viability after impact on day 1 (A) and day 4 (B) relative to core region. Values reported are averages ± standard deviations.
Finally, cores harvested from peripheral regions of the condyles, regions where cartilage is usually above the meniscus, had a statistically significant greater area of cell death (26.7%) than cores from the central (weightbearing) regions (11.2%; P = .01) even though there was no difference in thickness between groups (P = 1.0).
DISCUSSION
In this study, the distribution of chondrocyte death throughout cartilage thickness was investigated after impact injury. We showed progressive and expanding chondrocyte death after impact injury at energies insufficient to fracture the articular surface. In addition, an energy-dependent increase in total area of chondrocyte necrosis was observed in intact, impacted cores. Further, thinner cartilage and cartilage from peripheral regions of the condyles were more susceptible to impact injury.
In vivo experiments in animal models have shown that blunt injury to articular cartilage contributes to chondrocyte death and degenerative changes.3,21 Additionally, in vitro studies using a variety of cartilage-loading models show that chondrocyte death is a key initial event in post–joint injury degeneration.2,14,23 In the current study, we show an energy- and time-dependent progressive increase in chondrocyte necrosis after impact injury. We also show that while the distribution of dead chondrocytes is similar after low-, moderate-, and high-energy impact loading, the size of the dead zone is proportional to impact energy.
After high-energy impact to articular cartilage, Milentijevic et al18 showed extensive chondrocyte death throughout full cartilage thickness. They concluded that this phenomenon results from death extending to the deeper layers from the superficial zone as energy increases. This study showed increased chondrocyte death both at the site of impact (on the surface) and in the deep basal zone of the cartilage (at the subchondral bone/cartilage interface). These data suggest that chondrocyte necrosis observed after impact may additionally result from progressive deep-zone chondrocyte death with increasing impact energy and increasing time after impact.
The transitional interface between bone and cartilage, 2 different tissues with unique material properties,24 appears to be a focal region for increased cell death in this study. This injury pattern provides potential explanation for clinical observations of full-thickness chondral delamination occurring several months after anterior cruciate ligament injury. Because of limitations of in vitro study, it is possible that this observation is due to nonphysiologic loading conditions resulting from the limited amount of subchondral bone attached to the cartilage in this study. Although such limitations must be considered when interpreting the results, the use of animal tissues in vitro permits use of sufficient quantities of uniformly healthy cartilage to study the effects of impact injury on articular chondrocytes in situ.
Similar to this study, Chen et al6 employed in vitro study to show an increase in nonviable cells from 1% up to 74% over 2 days after impact injury. These data highlight the need to identify a treatment window for chondro-protective therapy. Compounds like cyclooxygenase inhibitiors,13 nonionic surfactant (P188),19 or derivative of glucosamine (Glu5)11 have been shown to reduce chondrocyte death if used shortly after the impact to articular cartilage. The data from the current study provide additional support to potential use of chondroprotective agents immediately after joint injury as a strategy to delay or prevent the onset of osteoarthritis.1,11,13,17,19
Pertinent to understanding potential risk factors for osteoarthritis, this study also showed that the extent of cartilage damage and/or death varied with cartilage thickness. Thinner cartilage cores fractured at lower energy levels, suggesting that individuals with thinner cartilage are at greater risk for severe impact injuries to the joint than are those with thicker cartilage under similar impact conditions. As these types of injury are often sustained during sporting activities,27 active women as a group may be at greater risk after impact injury as they tend to have thinner cartilage and an overall higher incidence of osteoarthritis.22,29
In summary, consistent patterns of progressive and expanding chondrocyte death were described after impact loading inadequate to grossly damage the articular surface. Cell death increased with increasing impact energy and progressed/enlarged over time. Moreover, the central regions of the condyles were more resistant to cellular injury. This study also showed that thinner articular cartilage fractures at lower impact energies. These data support a potential need for the use of chondroprotective compounds immediately after joint injury as a strategy to delay or prevent the onset of posttraumatic osteoarthritis. Such treatments may be especially important for individuals with thinner articular cartilage or in populations with greater incidence of osteoarthritis.
ACKNOWLEDGMENT
This study was funded by the National Institutes of Health grants R01 AR052784 (Dr Chu) and P60 AR054731 (Dr Chu), the Pittsburgh Foundation (Dr Szczodry and Dr Chu), and the Albert Ferguson Endowed Chair (Dr Chu).
Footnotes
REFERENCES
- 1.Baars DC, Rundell SA, Haut RC. Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech Model Mechanobiol. 2006;5(2-3):133–139. doi: 10.1007/s10237-006-0024-3. [DOI] [PubMed] [Google Scholar]
- 2.Borrelli J, Jr, Ricci WM. Acute effects of cartilage impact. Clin Orthop Relat Res. 2004;423:33–39. doi: 10.1097/01.blo.0000132627.13539.02. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli J, Jr, Silva MJ, Zaegel MA, Franz C, Sandell LJ. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. J Orthop Res. 2009;27(3):347–352. doi: 10.1002/jor.20760. [DOI] [PubMed] [Google Scholar]
- 4.Borrelli J, Jr, Tinsley K, Ricci WM, Burns M, Karl IE, Hotchkiss R. Induction of chondrocyte apoptosis following impact load. J Orthop Trauma. 2003;17(9):635–641. doi: 10.1097/00005131-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 6.Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001;19(4):703–711. doi: 10.1016/S0736-0266(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 7.Chu CR, Monosov AZ, Amiel D. In situ assessment of cell viability within biodegradable polylactic acid polymer matrices. Biomaterials. 1995;16(18):1381–1384. doi: 10.1016/0142-9612(95)96873-x. [DOI] [PubMed] [Google Scholar]
- 8.Dontchos BN, Coyle CH, Izzo NJ, et al. Optimizing CO2 normalizes pH and enhances chondrocyte viability during cold storage. J Orthop Res. 2008;26(5):643–650. doi: 10.1002/jor.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewers BJ, Dvoracek-Driksna D, Orth MW, Haut RC. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19(5):779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 10.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 11.Huser CA, Davies ME. Effect of a glucosamine derivative on impact-induced chondrocyte apoptosis in vitro: a preliminary report. Osteoarthritis Cartilage. 2008;16(1):125–128. doi: 10.1016/j.joca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Huser CA, Davies ME. Validation of an in vitro single-impact load model of the initiation of osteoarthritis-like changes in articular cartilage. J Orthop Res. 2006;24(4):725–732. doi: 10.1002/jor.20111. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey JE, Aspden RM. Cyclooxygenase inhibition lowers prostaglandin E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitro. Arthritis Res Ther. 2007;9(6):R129. doi: 10.1186/ar2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322(1):87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 15.Jones CW, Smolinski D, Keogh A, Kirk TB, Zheng MH. Confocal laser scanning microscopy in orthopaedic research. Prog Histochem Cytochem. 2005;40(1):1–71. doi: 10.1016/j.proghi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JL, Deloria LB, Oyen-Tiesma M, Thompson RC, Jr, Ericson M, Oegema TR., Jr Cell death after cartilage impact occurs around matrix cracks. J Orthop Res. 2003;21(5):881–887. doi: 10.1016/S0736-0266(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 17.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43(3-4):517–521. [PubMed] [Google Scholar]
- 18.Milentijevic D, Rubel IF, Liew AS, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19(7):466–473. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 19.Natoli RM, Athanasiou KA. P188 reduces cell death and IGF-I reduces GAG release following single-impact loading of articular cartilage. J Biomech Eng. 2008;130(4):041012. doi: 10.1115/1.2939368. [DOI] [PubMed] [Google Scholar]
- 20.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36(5):780–792. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 21.Newberry WN, Mackenzie CD, Haut RC. Blunt impact causes changes in bone and cartilage in a regularly exercised animal model. J Orthop Res. 1998;16(3):348–354. doi: 10.1002/jor.1100160311. [DOI] [PubMed] [Google Scholar]
- 22.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15(6):666–672. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19(2):242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 24.Radin EL, Paul IL, Lowy M. A comparison of the dynamic force transmitting properties of subchondral bone and articular cartilage. J Bone Joint Surg Am. 1970;52(3):444–456. [PubMed] [Google Scholar]
- 25.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am. 1977;59(8):1068–1076. [PubMed] [Google Scholar]
- 26.Rundell SA, Baars DC, Phillips DM, Haut RC. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23(6):1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 27.Saxon L, Finch C, Bass S. Sports participation, sports injuries and osteoarthritis: implications for prevention. Sports Med. 1999;28(2):123–135. doi: 10.2165/00007256-199928020-00005. [DOI] [PubMed] [Google Scholar]
- 28.Scott CC, Athanasiou KA. Mechanical impact and articular cartilage. Crit Rev Biomed Eng. 2006;34(5):347–378. doi: 10.1615/critrevbiomedeng.v34.i5.10. [DOI] [PubMed] [Google Scholar]
- 29.Slemenda CW. The epidemiology of osteoarthritis of the knee. Curr Opin Rheumatol. 1992;4(4):546–551. [PubMed] [Google Scholar]
- 30.Torzilli PA, Grigiene R, Borrelli J, Jr, Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121(5):433–441. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- 31.Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35(12):2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]