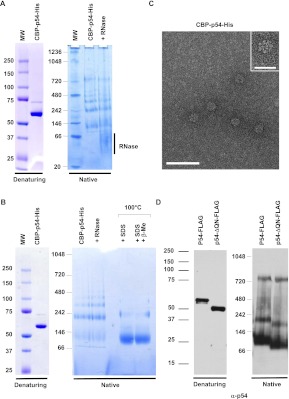
FIGURE 2.
RNA-independent oligomerization of recombinant Rck/p54 in vitro. (A) Oligomerization of purified CBP-p54-His. A total of 2 μg of purified recombinant CBP-p54-His was separated on denaturing (left) and native (right) gels, and stained by Coomassie. Where indicated, the protein was treated with RNase A prior to migration. (B) Aberrant migration of Rck/p54 in native gels. A total of 2 μg of purified recombinant CBP-p54-His was separated on denaturing (left) and native (right) gels, stained with Coomassie. Where indicated, the protein was treated with RNase prior to purification, or heated in the presence of SDS with or without β-mercaptoethanol prior to migration. (C) Visualization of the purified protein in electron microscopy. The protein preparation analyzed in A was spread on grids and stained with uranyl acetate prior to observation by electron microscopy in filtered zero loss mode. (Main panel) Magnification ×151,000; bar, 50 nm. (Inset) Magnification ×241,500; bar, 20 nm. (D) Oligomerization is independent of the QN-rich extension of the protein. A total of 50 ng of purified recombinant FLAG-tagged wild-type (p54-FLAG) and truncated (p54-ΔQN-FLAG) Rck/p54 proteins were separated on denaturing (left) and native (right) gels, and analyzed by Western blotting with anti-p54 antibodies.