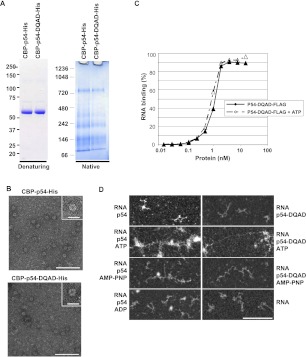
FIGURE 7.
RNA relaxing in the presence of Rck/p54 is independent of ATP hydrolysis. (A) Oligomerization of Rck/p54 DQAD mutant. A total of 2 μg of purified recombinant CBP-p54-His proteins, with or without an E/Q mutation in the DEAD motif, were separated on denaturing (left) and native (right) gels as in Figure 2A. (B) Visualization of Rck/p54 DQAD protein in electron microscopy. Both wild-type and mutant CBP-His-tagged proteins were visualized in electron microscopy as in Figure 2C. (Main panels) Magnification ×69,100; bar, 100 nm. (Insets) Magnification ×151,000; bar, 20 nm. (C) RNA-binding properties of Rck/p54 DQAD mutant. RNA binding was measured in the presence and absence of ATP using FLAG-tagged protein and filter retention assay, as in Figure 5C. (D) Electron microscopy imaging of RNA bound to Rck/p54 in the presence of AMP-PNP, or to Rck/p54 DQAD mutant. RNA was incubated with a 40 molar excess of the double-purified wild-type or mutant CBP-His-tagged proteins in the absence or presence of ATP, AMP-PNP, or ADP, as indicated. The mixture was spread and observed as in Figure 6. RNA alone is also shown. Magnification ×69,100; bar, 100 nm.