Abstract
Background: Argentina’s population was heavily affected by the 2009 influenza pandemic, particularly children, in whom incidence of seasonal influenza is consistently high. Following the pandemic, Argentinean national recommendations for pediatric vaccination against A/H1N1 influenza were defined for all children aged up to five years, in line with programs implemented by national authorities elsewhere. Economic evaluations have found that vaccination programs for this population against seasonal influenza are cost-effective, if not cost-saving in many countries. Recently, Argentina decided to routinely vaccinate against influenza children aged 6–23 mo-old. But, the economic value of such strategies for the country has never been assessed.
Methods: A model was developed to assess the value of four different vaccination strategies: (1) no pediatric vaccination; (2) vaccination of 6–23 mo-old children; (3) vaccination of 6–36 mo-old children; (4) vaccination of 6 mo−5 y-old children. We first estimated community health benefits of vaccination then we evaluated the economic and quality-of-life impact of these strategies on the population. Data used in the model come from surveillance networks, published literature, national databases and retrospective hospital-based data.
Results: Pediatric influenza vaccination benefited not only children but also the overall community, due to decreased disease transmission. Our results showed that the recent decision by Argentina to vaccinate 6–23 mo-old children is cost-effective as would be the incremental vaccination of broader age groups.
Conclusions: Results from this study are consistent with previous analyses in other countries confirming that implementing influenza pediatric vaccination programs can be highly cost-effective through individual- and community protection against the disease.
Keywords: influenza, vaccination, pediatrics, transmission, Argentina
Introduction
Over the last decade, pediatric vaccination against influenza increasingly has become part of national immunization policies. In the US, pediatric influenza vaccination was included in the national immunization program for children aged 6 to 23 mo in 2004, expanded to all children aged six to 59 mo of age in 2006, and then finally up to 18 y In Ontario, Canada, a universal influenza immunization program was set up in 2000, while in Taiwan, influenza vaccination policy targeted children aged 6 to 23 mo in 2004, followed by a school-based program in 2007. In Finland, influenza vaccination for children aged 6 to 35 mo has been fully subsidized since 2007. In Mexico, a pediatric influenza vaccination program was implemented gradually between 2004 and 2006 for populations aged up to 35 mo, and lastly a recent decision has been made in Columbia to vaccinate children under 2 y old.
Health authorities’ decisions to implement such pediatric vaccination programs are based upon several factors including an estimation of the potential economic and public health benefits for vaccinated children as well as for other community members.1 The individual burden of influenza in children is not limited to the viral infection itself but is also linked to complications of the illness, such as otitis media, pneumonia and exacerbation of pulmonary diseases such as bronchitis and asthma, which frequently develop in very young children.2,3 Children also contribute significantly to virus transmission4-6 since the contact patterns of children tend to be inter-generational7 they tend to act as a “backbone of transmission” in the family and, consequently, in the general population.8
Vaccination is the most effective measure for preventing influenza. The results of both empirical and modeling studies have shown that pediatric vaccination programs in school and day-care settings can reduce the influenza attack rate in families of vaccinated children and in the community,4,9 and that vaccination of 50% to 70% of children could help in containing annual influenza epidemics.10-12 Health economic evaluations conducted previously have found influenza vaccination programs targeting children to be at the least cost-effective and very often cost-saving, due to disease prevention, particularly when impact on transmission had been considered.8,13
Argentina was one of the countries in South America most affected by the 2009 pandemic of A/H1N1 virus (pH1N1), with cases seen as early as May 2009. The A/H1N1 2009 disease burden exerted a substantial impact on the operational capacities of local health care systems. School-aged children in Buenos Aires and surrounding areas were affected first, with high rates of transmission. Later, other populations (mainly young adults, pregnant women and young children) were affected, and the infection spread to the general population of the metropolitan area, the Province of Buenos Aires and the rest of the country. A recent study in Argentina showed that death rate due to influenza for children during the pandemic was ten times higher than during previous influenza seasons.14 By early 2010, six months after the start of the pandemic spread, more than 1.4 million cases of influenza-like illness had been officially reported.15
Before the 2009 A/H1N1 pandemic, seasonal influenza vaccination was recommended and funded for Argentineans from six months of age having chronic comorbidities and the elderly population aged 65 y and over.2,16 During the pandemic, the Argentinean Ministry of Health recommended influenza vaccination for all children less than five years of age. It has been demonstrated that improved public awareness and strong recommendations are key to increasing vaccination coverage rates.17 Accordingly, in 2010, Argentinean vaccination against influenza is expected to increase due to greater awareness generated by the recent pandemic.
The objective of the present study was to show if the recent routine vaccination for children from 6 to 23 mo is cost-effective and to evaluate the public health and economic impact of allocating public funds to broader pediatric groups in Argentina, taking into consideration the reduction of the transmission from children to the general population.
Results
Results are presented for the entire 2006 Argentinean population of 40.7 million.18
Public health impact of vaccination scenarios
The numbers of people vaccinated, developing influenza and dying due to influenza are reported for the different vaccination strategies in Table 5. Compared with baseline, the vaccination program targeting the 6 mo−5 y-old population was associated with a 9.39% reduction (435,569 fewer cases) in influenza cases among the general population, compared with a 5.17% reduction (239,844 fewer cases) for the 6–36 mo-old strategy and a 2.43% (112,649 fewer cases) reduction for the 6–23 mo-old strategy. Among the unvaccinated population, the attack rate decreased from 11.80% (baseline) to 11.56% (6–23 mo), 11.33% (6–36 mo) and 10.83% (6 mo−5 y-old) due to herd immunity effects simulated in the model.
Table 5. Number of subjects vaccinated, ILI cases and ILI-related deaths for each scenario using base case inputs and stepwise comparisons (presented as a percentage difference*) for each vaccination strategy vs. the next narrowest strategy.
| Vaccinated | ILI cases | ILI related death | |
|---|---|---|---|
|
Baseline |
4,115,399 |
4,639,133 |
8,430 |
|
6–23 mo |
4,583,058 |
4,526,484 |
8,330 |
|
6–23 mo vs. baseline |
11.36% |
-2.43% |
-1.18% |
|
6–36 mo |
5,201,181 |
4,399,289 |
8,122 |
|
6–36 mo vs. 6–23 mo |
13.49% |
-2.81% |
-2.49% |
|
6 mo–5 yo |
5,807,105 |
4,203,563 |
7,778 |
| 6 mo–5 y old vs. 6–36 mo | 11.65% | -4.45% | -4.24% |
Percentage difference is calculated as the difference between two numbers, expressed as a percentage of one of the numbers. For example, for 6–23 mo vs. baseline, percentage difference in number vaccinated (11.36%) = [number vaccinated with the 6–23 mo strategy (4,583,058)–number vaccinated with the baseline strategy (4,115,399)]/number vaccinated with the baseline strategy.
Pair-wise comparisons of public health benefits linked to each vaccination strategy, vs. the next narrowest strategy, are presented in Table 5. In terms of influenza cases, the 6 mo–5 y-old strategy is associated with a 4.45% reduction vs. the 6–36 mo-old strategy, the 6–36 mo-old vaccination strategy is associated with a 2.81% reduction vs. the 6–23 mo-old strategy and the 6–23 mo-old strategy is associated with a 2.43% reduction vs. baseline, demonstrating that each more extensive vaccination strategy yields fewer cases of influenza than the narrower one.
Economic and cost-effectiveness results
Incremental QALYs, costs and ICERs, relative to baseline, were reported for the various vaccination strategies in Table 6. Compared with the baseline situation, investment in vaccination programs resulted in ICERs of $1,759, $1,103 and $717 per QALY for the 6–23 mo, 6–36 mo-old and 6 mo–5 y-old strategies, respectively. ICERs for the three vaccination strategies were far below the threshold of $8,100 per QALY and decreased as vaccination coverage expanded.
Table 6. Results from cost effectiveness analysis and from deterministic sensitivity analysis varying ILI incidence and effectiveness against ILI: QALYs gained, costs in US dollars (US$) 2009 and the ICER for each vaccination strategy in comparison with baseline.
| Vaccination strategy |
Differences vs. baseline |
||
|---|---|---|---|
| QALYs gained | Incremental costs (US$) | ICER | |
| 6–23 mo |
2,092 |
$3,680,050 |
$1,759 |
| 6–36 mo |
5,267 |
$5,807,474 |
$1,103 |
| 6 mo–5 yo | 10,505 | $7,534,987 | $717 |
| ILI incidence* | Low | High | Low | High | Low | High |
|---|---|---|---|---|---|---|
| 6–23 mo |
2,921 |
3,920 |
$3,591,434 |
$2,355,696 |
$1,229 |
$601 |
| 6–36 mo |
5,665 |
12,310 |
$5,898,280 |
$2,551,782 |
$1,041 |
$207 |
| 6 mo–5 y old |
9,626 |
21,403 |
$7,735,865 |
$2,570,285 |
$804 |
$120 |
|
Vaccine effectiveness* |
Low |
High |
Low |
High |
Low |
High |
| 6–23 mo |
1,672 |
2,628 |
$3,677,287 |
$2,910,060 |
$2,200 |
$1,107 |
| 6–36 mo |
4,353 |
8,046 |
$6,705,691 |
$4,283,312 |
$1,540 |
$532 |
| 6 mo–5 y old | 9,922 | 11,360 | $7,556,446 | $6,423,463 | $762 | $565 |
Cost-effectiveness pair-wise comparisons of each strategy are provided in Table 7. The 6–23 mo-old strategy was less costly but less effective than the other two strategies, the 6–36 mo-old strategy was cost-effective vs. the 6–23 mo-old strategy (ICER of $670 per QALY) but less costly and less effective than the 6 mo–5 y-old strategy and the 6 mo–5 y-old strategy was cost-effective vs. the two other strategies (ICERs of $458 per QALY vs. the 6 mo–23 mo-old strategy and $330 per QALY vs. the 6 mo-old–36 mo-old strategy).
Table 7. Cost-effectiveness incremental comparisons of each vaccination strategy.
| Vaccination strategy |
Compared with: |
|||
|---|---|---|---|---|
| Baseline | 6–23 mo | 6–36 mo | 6 mo–5 y old | |
|
6–23 mo |
$1,759 |
- |
Less costly, less effective |
Less costly, less effective |
|
6–36 mo |
$1,103 |
$670 |
- |
Less costly, less effective |
| 6 mo–5 y old | $717 | $458 | $330 | - |
Sensitivity analyses
The most impacting variables were vaccine effectiveness and influenza attack rates, shown in Table 6. In the optimistic scenario (largest coverage; highest influenza incidence; 6 mo-old–5 y-old vaccination strategy) more than 20,000 QALYs were gained at an incremental cost of $120 per QALY. Conversely, the pessimistic scenario (low vaccine effectiveness; low vaccine coverage; 6–23 mo-old vaccination strategy) was associated with a gain of approximately 1,700 QALYs at an incremental cost of $2,200 per QALY. All ICERs obtained in the DSA were below the Argentinean yearly GDP per capita ($8,100).
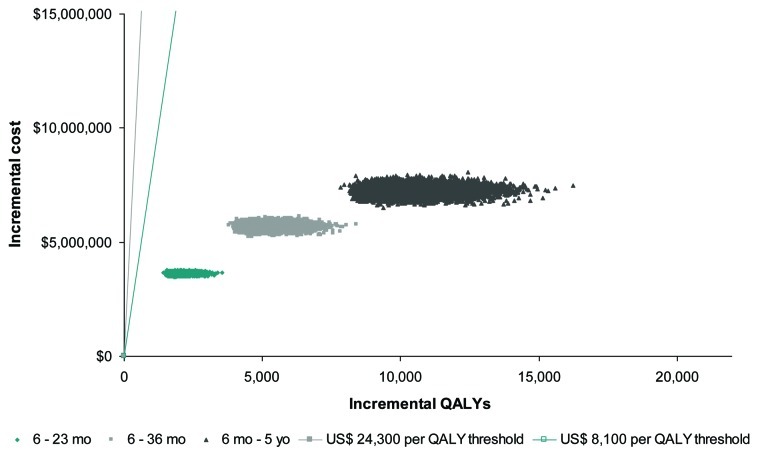
Probabilistic sensitivity analysis results are presented on a cost-effectiveness (CE) plane in Figure 2. The CE plane compares the results of 10,000 simulations with willingness-to-pay thresholds of 1 × GDP ($8,100) and 3 × GDP ($24,300). One hundred per cent of the simulations conducted fell below both thresholds, demonstrating that each of the pediatric vaccination strategies tested in this model can be considered to be very cost-effective and that the results of the economic evaluation as robust.
Figure 2.
Cost-effectiveness plane of PSA. All vaccination scenarios are below cost-effectiveness thresholds of $8,100 (US) and $24,300 (US) per QALY.
Discussion
The primary objective of the present study was to estimate the consequences of implementing pediatric vaccination programs against influenza in Argentina, taking into account the individual and community benefits of vaccinating children.
Three pediatric vaccination scenarios, each one simulating the vaccination of a larger group of children, were tested and compared with a strategy with no pediatric influenza vaccination program. The benefits of each scenario were also compared incrementally. Vaccinating children aged six months to five years of age was associated with the lowest number of episodes of influenza in the population but also was found to be the most cost-effective. This strategy was associated with the lowest ICER vs. baseline and was always cost-effective vs. the other vaccination strategies. It demonstrates that the more children are vaccinated, the more population can benefit from indirect protection.
Our results show that vaccinating older age groups increases the cost-effectiveness of the immunization strategy. This effect can be due to a higher impact of vaccination in some older groups but also to the nonlinear impact of indirect protection. The more individuals are vaccinated, in this case children, the more difficulties the virus will have to spread in the population. Hence, unvaccinated individuals benefit also from those strategies as shown in Figures 3 and 4.
Figure 3.
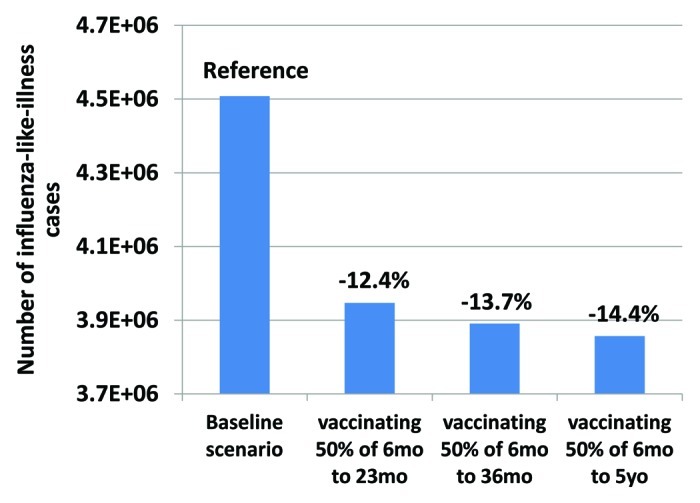
Number of cases in unvaccinated individuals. Compared to the baseline scenario, all pediatric vaccination scenarios show a reduction in the number of cases in unvaccinated individuals in the general population.
Figure 4.
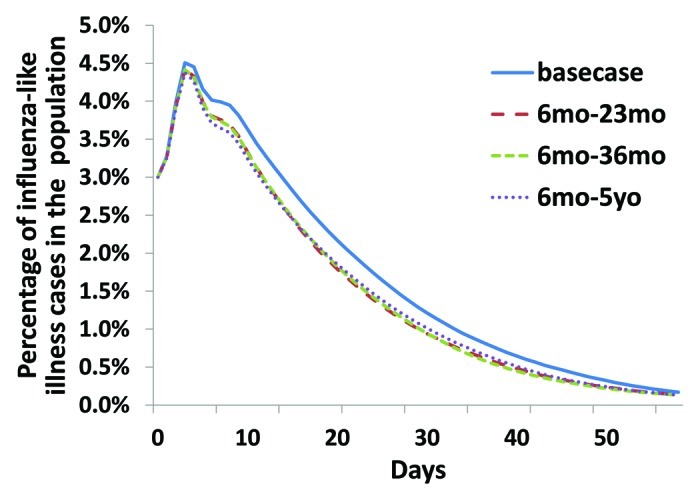
Temporal evolution of the number of infected individuals. For the four different situations, we averaged epidemic curves over 1000 simulations. We can notice the impact of pediatric vaccination on the course of the epidemic. However our model is not able to differentiate the temporal impact of the different pediatric strategies over the period of time considered.
One limitation of this study was the lack of Argentina-specific data. Medical and demographic differences could have an impact on factors such as the likelihood of receiving medical treatment for influenza or to being admitted to hospital, and some of the assumptions on resource use were derived from international sources. In particular, no Argentinean specific data on health care consumption and health care performance were available, which could affect the probability of visiting a GP for influenza or even the probability of dying from influenza. The decrease of quality of life during an influenza episode is also a very uncertain parameter which could vary from country to country. A wide range of estimates of this score have been published in the literature.19 The estimate we use is in agreement with more recently published ones20 but slightly more conservative since the associated quality of life decrease is lower. Furthermore, although the yearly variability of influenza circulation was partly considered in the current model by averaging influenza attack rates over several years, it is worth noting that the benefit of one vaccination program will also vary with the pathogenicity and the contagiousness of the circulating virus strain as well as the concordance with the strains contained in the vaccine (predicted by WHO). Because of lack of laboratory confirmed influenza surveillance data, we had to rely on influenza-like-illness reported cases. Influenza-like-illnesses are generally a good approximation of influenza epidemic trend but they are due to a wide range of sources of infection. However it was accounted for in the vaccine efficacy data we used and should not bias our results. Deterministic and probabilistic sensitivity analysis produced results consistent with the conclusions from the base case results. More specifically, it showed that a high influenza incidence combined with high vaccine effectiveness give better cost-effectiveness ratios than low incidence and low vaccine effectiveness. No simulated ICERs, for any vaccination strategy, were above $8,100 per QALY threshold; all three strategies were very cost-effective in 100% of the simulations conducted. Our assumptions are relatively conservative compared with other cost effectiveness analysis of pediatric influenza vaccination. For example, Dayan et al.2 used a vaccine effectiveness of 70% and an attack rate of 25%.
Consequently we think that our results are also more conservative that previous results obtained from various pediatric studies. A recent review13 showed that 9 out of 10 studies showed that pediatric vaccination was cost saving. In our analysis, although we do not show cost saving ratios, we demonstrate that pediatric vaccination is very cost effective and that a step by step approach in terms of targeted population, may be a viable approach as it has been done in other countries (US, Canada, Mexico…). Incrementally expanding the vaccination range has the advantage of easing the logistical implementation and the funding process. We showed in our study that each recommendation expansion is cost-effective compared with the previous one.
Conclusion
In conclusion, expanded vaccination strategies in pediatrics represent a cost-effective use of healthcare resources in Argentina. In terms of both clinical and cost-effectiveness targeting the 6 mo–5 y-old for vaccination, even with a step by step approach, represents the best use of public funding, in that it yields the lowest ICER and the lowest incidence of influenza infections. The current decision of vaccinating children from 6 mo-old to 2 y-old seems to be a step in the right direction.
Materials and Methods
We developed a model which combines: (1) an epidemiological simulation estimating public health benefits in terms of influenza cases and deaths prevented by pediatric vaccination; (2) a cost-effectiveness simulation balancing vaccination costs with savings and improvements in quality-of-life due to disease averted.
Epidemiological modeling
We used the stochastic model introduced by Halloran and colleagues11 to study influenza transmission and influenza disease prevention strategies within a synthetic population. We defined the simulated population to be representative of a typical Argentinean community in terms of age distribution21 and household composition22 Halloran’s model was based on work by Elveback and colleagues,10 who originally developed a structured population model to analyze person to person influenza transmission during epidemics. As in previous studies, our model simulates daily interactions at different levels between persons living in the same household, persons belonging to different activity groups according to age, and persons living in the same neighborhood and community. The model has a hierarchical structure, in which individuals belong to households located in a neighborhood. A community is composed of four neighborhoods and five communities live along one another and share common workplaces. Upon that structure, the model simulates the spread of influenza infection and cases in the overall population through the computation of individual probabilities of infection depending on the number of infected individuals in the different groups. Influenza is introduced into the population by a small number of “initial infectives,” who spread the infection in the population. Based on their infection status, individuals can be in one the following states: susceptible, exposed, infectious (symptomatic or asymptomatic), or immune. Probabilities of transition from a susceptible to an exposed individual are computed in the model based on the number of infected in contact with the individual in its household and in its activity groups. Duration of stay in the exposed and infectious states is stochastically computed based on data from previous studies10,11 Infection probabilities are derived from real data generated in the Monto et al. study.5 Individuals who become infected as a result of contact with an infective may themselves spread the disease to other susceptible persons among their contacts and so on (Fig. 1A).
Figure 1.
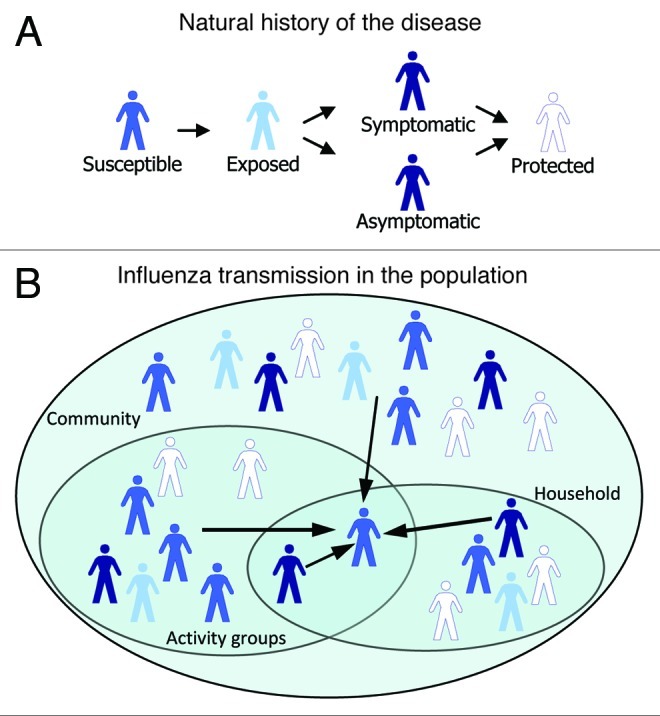
(A) natural history of influenza in the model. Individuals are in various health states. From susceptible, they become exposed, then they develop symptoms or remain asymptomatic, and finally they become immune to the disease. (B) Influenza transmission in the population. Arrows pointing to an individual stand for probabilities of infection which depend on the number of infectious individuals in his/her activity group, household, neighborhood and community.
With reference to the activity groups, toddlers (6–23 mo) attend day-care centers or stay at home; all children of pre-school age (24–59 mo) attend either a small playgroup or large day-care center; school-aged children (5–18 y) attend a school. Working adults are assigned to workplaces where they can mix with other adults belonging to other communities. During the simulation, all persons in the population come into contact, on a daily basis, with members of their households and of the various activity or social groups to which they belong (i.e., playgroup, day-care center, school, workplace, neighborhood and community) (Fig. 1B).
Selected children and adults in the model are designated at “high-risk” of complications (vs. “low-risk”). The proportion of high-risk individuals is the estimated percentage of persons within an age group with chronic co-morbidities (e.g., cardiac, respiratory or metabolic) that could render them more susceptible to influenza-related complications than the general healthy population.
Vaccination is considered to have been implemented prior to the start of the influenza season. Infectiousness of an infected individual depends upon his/her vaccination status, whether the infection is in a latent or infective stage, and whether clinical illness has developed. An individual’s susceptibility to infection depends only upon vaccination status. Vaccination, therefore, is observed to have a direct epidemiological effect in reducing susceptibility to infection among vaccinated individuals and an indirect effect on influenza transmission by reducing the number of infective individuals in the community, thus reducing the epidemic attack rate.
The model is calibrated to reflect the average influenza-like illness (ILI) attack rate observed in Argentina by the syndromic influenza surveillance network over several influenza seasons (Table 1).23 Consequently, we used in our computations vaccine effectiveness against ILI and not against laboratory confirmed influenza (Table 3). Details of the calibration are given in Appendix 2.
Table 1. Incidence of symptomatic influenza (influenza-like-illness; ILI) in the Argentinean population average over 2002 to 2006 seasons23.
| Age group of general population |
Incidence of ILI |
||
|---|---|---|---|
| Min | Average | Max | |
|
< 5 y |
7.47% |
9.73% |
12.49% |
|
5–49 y |
11.64% |
12.63% |
15.21% |
| ≥ 50 y | 3.99% | 5.61% | 7.71% |
Table 3. Average vaccine efficacies against influenza like illness per age groups.
Cost-effectiveness analysis
Each case of influenza identified in the epidemiological part of the model is attributed a specific probability of:
consuming ambulatory medical resources for treating influenza or its complications
being hospitalized for treating influenza or its complications
dying due to influenza
Decreases in quality-adjusted life-years (QALYs) due to influenza, influenza complications or premature deaths, are subsequently estimated for each individual. Life years and future costs were discounted at a rate of 3% per year.
The following vaccination scenarios were explored:
Baseline scenario: no specific public funding allocated to pediatric vaccination, which corresponds to the pre-2009-pandemic situation,
Public vaccination program covering 50% of children six months old to 23 mo old (6–23 mo), which corresponds to the 2011 recommendation,
Public vaccination program covering 50% of children six months old to 36 mo old (6–36 mo),
Public vaccination program covering 50% of children six months old to five years old (6 mo-old–5 y old).
For each vaccination program, the economic metric considers (1) public investment for pediatric influenza vaccination (vaccine purchase, vaccine administration costs and costs of adverse events); (2) Direct medical outpatient (drug- and physician visit-related costs) and inpatient costs related to the treatment of the remaining cases of influenza in the whole population. Indirect costs were not considered in this analysis.
Outputs
The model estimates the public health and economic consequences linked to each tested vaccination program. To compare the consequences of the four different strategies, the model calculates their incremental cost-effectiveness ratios (ICERs), i.e., the investment needed to gain one additional QALY relative to the current situation. Each vaccination scenario is also compared with the two others, assuming extension of recommendations from 6–23 mo-old to 6 mo–5 y-old (the 6 mo–5 y-old strategy is compared with the 6–36 mo-old strategy, the 6–36 mo-old is compared with the 6–23 mo-old strategy and the 6–23 mo-old strategy is compared with baseline).
ICERs were compared with the annual Argentinean GDP per capita24 and to three times this amount ($24,300), which is the willingness-to-pay threshold proposed by the World Health Organization (WHO) when no other acceptability threshold is defined locally. We used the 2008 estimate from the World Bank which gives a GDP per capita of US $8,100.
Input data
Data were obtained from literature reviews and official reports from the Argentinean Ministry of Health. When no sources could be identified, the authors made assumptions based upon data in other countries or personal experience. Within each age group, most data were stratified according to the presence of chronic comorbidities (i.e., according to the risk group).
Parameters related to the epidemiological model
A detailed list of inputs related to population parameters used in the model is provided in Table 2. Population structure parameters were taken from Argentinean demographic data. Other inputs concerning workgroup characteristics not available specifically for Argentina are general assumptions from previously published modeling studies.8,10 The proportion of high-risk individuals in the Argentinean population is an estimate based on a previous study specific to high-risk groups for the 0–18 y old age group and are assumptions for the other age groups.
Table 2. Demographic parameters.
| Population inputs |
Value |
Source |
|---|---|---|
| Distribution of age groups* | ||
| 0–6 mo old |
1% |
Estadisticas vitales—informacion basica año 2006. Ministerio de salud de la nación21 |
| 6–23 mo old |
3% |
|
| 2–4 y old |
5% |
|
| 5–10 y old |
9% |
|
| 11–14 y old |
9% |
|
| 15–18 y old |
9% |
|
| 19–49 y old |
42% |
|
| 50–64 y old |
13% |
|
| 65+ y old | 10% | |
| Distribution of household size (in persons)* | ||
|---|---|---|
| 1 |
16% |
Estadisticas vitales—informacion basica año 2006. Ministerio de salud de la nación21 |
| 2 |
21% |
|
| 3 |
19% |
|
| 4 |
19% |
|
| 5 |
13% |
|
| 6 |
7% |
|
| 7 |
4% |
|
| Probability of monoparental household |
0.18 |
22 |
| Probability of household with children and elderly |
0.08 |
22 |
| Average size of workgroups (in persons) |
25 |
8, 10 |
| Probability of working in a different community |
0.6 |
8, 10 |
| Proportion of workers in the active population | 0.922 | 22 |
| Proportion of high-risk individuals in each age group | ||
|---|---|---|
| 0–18 y old |
5% |
Assumptions |
| 19–64 y old |
30% |
|
| 65+ y old | 40% | |
total does not match 100% due to rounding.
Vaccination program
Vaccine efficacies were determined from the literature, taking into account the fact that influenza attack rates used to calibrate the model were estimated from symptomatic cases of influenza (influenza-like illness; ILI) collected through the Argentinean sentinel network.23 Vaccine efficacies against ILI for each age group are provided in Table 3. Vaccine coverage rates of high- and low-risk individuals are listed in Table 4. In the model, current seasonal influenza vaccine coverage rates were assumed to be 5% of those aged 0–4 y (12% for high risk people), 1% of those aged 5–18 y (6% for high risk people), 10% of those aged 19–64 y (16% for high risk people) and 31% of those aged 65 y and over. It was assumed that the organization of a pediatric vaccination program would allow vaccination of 50% of targeted children.
Table 4. Vaccine coverage rates of low- and high-risk people for each scenario.
| Vaccine coverage of low-risk/high-risk people | ||||
|---|---|---|---|---|
| Baseline | Scenario 1: 6 mo–23 mo | Scenario 2: 6 mo–36 mo | Scenario 3: 6 mo–5 yo | |
| 0–6 mo old |
0.0%/0.0% |
|||
| 6–23 mo old |
4.8%/12.0% |
50.0%/50.0% |
50.0%/50.0% |
50.0%/50.0% |
| 2–4 y old |
4.8%/12.0% |
4.8%/12.0% |
35.0%/37.3% |
50.0%/50.0% |
| 5–10 y old |
0.8%/6.4% |
0.8%/6.4% |
0.8%/6.4% |
9.0%/14.0% |
| 11–18 y old |
0.8%/6.4% |
|||
| 19–64 y old |
9.6%/16.0% |
|||
| 65+ y old | 31.2%/31.2% | |||
For vaccinated individuals, the probability of vaccine adverse events is 1%;25 and adverse events are always assumed to require consultation with a physician.
Influenza-related resource use (details in Appendix 1)
For those who contract influenza, probabilities of GP consultations were based upon published literature: for low-risk patients, they were taken from three articles according to age group.26-28 For high-risk patients, probabilities of GP consultations were calculated as twice those for low-risk patients.29
Duration of a single influenza episode was estimated to be 4.1 d.8 It was assumed that 100% of patients suffering from influenza would make at least one purchase of over-the-counter medication and that a physician consultation for influenza would lead to the prescription of at least one pharmaceutical treatment.
For those who develop complicated influenza, probabilities of hospitalization for subjects aged 0–14 y were taken from an economic evaluation of influenza vaccination in children in Argentina.2 Probabilities for all other age-groups were taken from studies reported by Turner et al. in 2003.28
In the absence location-specific data, probabilities of death due to influenza were taken from a published economic evaluation of influenza vaccination in the European Union.30
Costs
All costs used in the model are in 2009 US dollars ($).40–42 Details of the unit costs used in the analysis are provided in Appendix 1.
Utilities
Quality-of-life was assumed to decrease during an influenza episode or during complications of influenza. The utility scores for influenza or influenza hospitalizations were taken from a US study of the management of influenza symptoms in healthy adults.31 Quality-of-life was set to “0” for subjects dying due to influenza. Years of life lost were quality-weighted according to the mean valuations of health for each age category, obtained from a EuroQol study in Argentina.32 Those aged less than 18 y were not included in the EuroQol study so, in the absence of other data, the utility score for those aged 19–49 y was conservatively applied to this age group in the model.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were conducted to identify the sources of uncertainty in the set of parameters. Probabilistic sensitivity analysis (PSA) simulations were run 10,000 times for each vaccination strategy to assess the impact of parameter uncertainty on the model outcomes. A deterministic sensitivity analysis (DSA) was specifically performed to assess the impact of vaccine effectiveness and yearly variations of influenza attack rates on the model outcomes. The lowest and highest values of attack rates observed among the five year-period considered to estimate Argentinean ILI incidence (2002–2006; see Table 1) were used as lower and upper bounds for the DSA. Lower and upper DSA values for vaccine effectiveness were given by averaging lower and upper bounds of confidence intervals provided in the publications from which influenza vaccine effectiveness was estimated (see Table 3). For both incidence and effectiveness DSA, the probabilities of infection in the epidemiological model were specifically calibrated to match the observed baseline under the new assumptions given by the scenario. The calibration process used an implementation of the simplex algorithm to estimate the right set of probabilities (source code available upon request). Details about remaining parameter values used in DSA and PSA are provided in Appendix 1.
Appendix 1. Values and sources used for the cost-effectiveness model. Costs are in US dollars (US$) 2009.
| Main model parameters |
Base case value |
Source |
Sensitivity analysis value |
Source |
|||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Life expectanciesa (years) | |||||||
| 0–5 mo |
74.40 |
21 |
Not varied in sensitivity analysis |
||||
| 2–4 y old |
72.52 |
||||||
| 5–10 y old |
68.16 |
||||||
| 11–14 y old |
63.26 |
||||||
| 15–18 y old |
59.33 |
||||||
| 19–49 y old |
42.55 |
||||||
| 50–64 y old |
22.16 |
||||||
| 65 + y old |
8.40 |
||||||
|
Influenza clinical consequences | |||||||
| Average duration of one influenza episode |
4.10 |
8 |
PSA: Gamma distribution, from 3 to 6 days SE = 1.51 |
||||
| Average number of GP consultationsb | |||||||
| |
Low-risk |
High-risk |
|
Low-risk |
High-risk |
|
|
| 0–5 mo |
0.90 |
1.80 |
|
DSA: 0.5–1.0 PSA: Triangular distribution SE = 0.13 |
DSA: –2.0 PSA: Triangular distribution SE = 0.26 |
Low-risk: Lower bound: 27 Upper bound: 2 High-risk: 2 x low-risk rates according to 27 |
|
| 6–23 mo |
0.70 |
1.40 |
DSA: 0.5–1.0 PSA: Triangular distribution SE = 0.13 |
DSA: –2.0 PSA: Triangular distribution SE = 0.26 |
|||
| 2–4 y old |
0.50 |
1.00 |
DSA: 0.47–1.0 PSA: Triangular distribution SE = 0.14 |
DSA: 0.94–2.0 PSA: Triangular distribution SE = 0.27 |
|||
| 5–10 y old |
0.28 |
0.56 |
DSA: 0.28–1.0 PSA: Triangular distribution SE = 0.18 |
DSA: 0.56–2.0 PSA: Triangular distribution SE = 0.37 |
|||
| 11–14 y old |
0.24 |
0.48 |
DSA: 0.24–1.0 PSA: Triangular distribution SE = 0.19 |
DSA: 0.48–2.0 PSA: Triangular distribution SE = 0.39 |
|||
| 15–49 y old |
0.09 |
0.18 |
DSA: 0.09–0.28 PSA: Triangular distribution SE = 0.05 |
DSA: 0.18–0.28 PSA: Triangular distribution SE = 0.03 |
Low-risk: Lower bound: 26 Upper bound: 28 High-risk: 2 x low-risk rates according to 29 |
||
| 50–64 y old |
0.09 |
0.18 |
DSA: 0.09–0.28 PSA: Triangular distribution SE = 0.05 |
DSA: 0.18–0.28 PSA: Triangular distribution SE = 0.03 |
|||
| 65 + y old |
0.33 |
0.65 |
DSA: 0.33–0.65 PSA: Triangular distribution SE = 0.08 |
DSA: 0.65–1.30 PSA: Triangular distribution SE = 0.17 |
Low-risk: Lower bound: 28 Upper bound: 2 x lower bound (assumption) High-risk: High-risk: 2 x low-risk rates according to 29 |
||
| Probability of hospitalizationsc | |||||||
| |
Low-risk |
High-risk |
|
Low-risk |
High-risk |
|
|
| 0–14 y old |
0.01 |
0.04 |
2 |
Not varied in sensitivity analysis |
|||
| 15–49 y old |
0.0002 |
0.0037 |
|
||||
| 50–64 y old |
0.0004 |
0.0044 |
|||||
| 65 + y old |
0.01 |
0.03 |
|||||
| Probability of death | |||||||
| |
Low-risk |
High-risk |
|
Low-risk |
High-risk |
|
|
| 0–49 y old |
0.0001 |
0.0001 |
30 |
Not varied in sensitivity analysis |
|||
| 50–64 y old |
0.0047 |
0.01 |
|||||
| 65 + y old |
0.01 |
0.01 |
|||||
|
Economic parameters (costs in US$) | |||||||
| Vaccination costsd | |||||||
| 6–23 mo |
$10.19 |
40 and private communication from Argentina Ministry of Health, Vaccine division |
Not varied in sensitivity analysis Not varied in sensitivity analysis |
||||
| 2–4 y old |
$6.47 |
Not varied in sensitivity analysis |
|||||
| 5 y old + |
$6.29 |
Not varied in sensitivity analysis |
|||||
| GP consultatione |
$5.25 |
41 |
Not varied in sensitivity analysis |
||||
| Prescription drugsf |
$1.83 |
Argentina market share data from IMS |
DSA: $1.56–$2.10 (+/− 15%) PSA: Gamma distribution SE = 0.14 |
Assumption |
|||
| OTC drugsf |
$2.70 |
Argentina market share data from IMS |
DSA: $2.30–$3.11 (+/− 15%) PSA: Triangular distribution SE = .21 |
Assumption |
|||
| Hospitalization costg | |||||||
| |
Low-risk |
High-risk |
|
Low-risk |
High-risk |
|
|
| 0–18 y old |
$1,387.05 |
$2,075.19 |
IRA database from Hospital de Ninos Ricardo Gutierrez & Nomenclador de prestacioned de salud de la ciudad de Buenos Aires (2008) |
DSA: $1,178.99–$1,595.11 (+/− 15%) PSA: Gamma distribution SE = 106.15 |
DSA: $1,763.91–$2,386.47 (+/− 15%) PSA: Gamma distribution SE = 158.82 |
Assumption |
|
| 19–49 y old |
$1,257.69 |
$1,257.69 |
DSA: $1,069.04–$1,446.34 (+/− 15%) PSA: Gamma distribution SE = 96.25 |
DSA: $1,069.04–$1,446.34 (+/− 15%) PSA: Gamma distribution SE = 96.25 |
|||
| 50–64 y old |
£1,437.36 |
$1,437.36 |
DSA: $1,221.76–$1,652.96 (+/− 15%) PSA: Gamma distribution SE = 110.00 |
DSA: $1,221.76–$1,652.96 (+/− 15%) PSA: Gamma distribution SE = 110.00 |
|||
| 65 + y old |
$1,886.54 |
$1,886.54 |
DSA: $1,603.55–$2,169.52 (+/− 15%) PSA: Gamma distribution SE = 144.38 |
DSA: $1,603.55–$2,169.52 (+/− 15%) PSA: Gamma distribution SE = 144.38 |
|||
|
Utilities | |||||||
| Utilities for general population | |||||||
| 0–5 mo |
0.89 |
32 |
Not varied in sensitivity analysis |
||||
| 6–23 mo |
0.89 |
||||||
| 2–4 y old |
0.89 |
||||||
| 5–10 y old |
0.89 |
||||||
| 11–14 y old |
0.89 |
||||||
| 15–18 y old |
0.89 |
||||||
| 19–49 y old |
0.82 |
||||||
| 50–64 y old |
0.92 |
||||||
| 65 + y old |
0.70 |
||||||
| Utilities in case of influenza |
0.25 |
31 |
Not varied in sensitivity analysis |
||||
| Utilities in case of hospitalizations | 0.20 | 31 | Not varied in sensitivity analysis | ||||
Appendix 1. (continued)aCalculated from Argentinean mortality rates. bRates for low-risk 0–3 y old come from expert opinion; 3–65+ y old come from literature: 3–17 y old;27 18–64 y old;26 65+y old.28 High-risk were calculated as 2 x low-risk rates as from literature.2c0–14 y old come from literature.2 15–64 y old were calculated from literature.28 Low-risk: mean of 0.44% for 65–74 y old and 1.13% for 75+ y old. High-risk: mean of 2.4% for 65–74 y old and 4.08% for 75+ y old. dIncluding vaccine cost and vaccine administration costs based on the following assumptions for vaccine schedule: children aged 6–18 mo receive two doses of 0.25 ml; children aged 19–36 mo receive one dose of 0.25 ml; those aged 36 mo+ receive one dose of 0.50 ml. ePublic GP consultation at the hospital is $5.25. As a minority of patients has private visits, only the public value is considered in the model. fWeighted average hospital (prescription)/private (OTC) costs of ibuprofen, dipirona and paracetamol, based upon Argentinean market share data from IMS 2009. gHospitalization cost = average number of hospitalized x daily hospitalization costs. For 0–18 y olds, mean number of hospitalized days come from Hospital de Niños Ricardo Gutiérrez IRA database (2002–2007) and was 7.72 for the low-risk and 11.55 days for the high-risk. For other age groups experts assume an average of 7 days for 18–50 y old, 8 days for 50–64 and 10.5 days for the 65+. Daily hospitalization cost of $179.67 comes from “Nomenclador de prestaciones de salud de la ciudad de Buenos Aires” (2008).
Appendix 2. Contact rates and probability of infection estimation.
| Probability of infection per group |
Base case value |
|---|---|
| probability of infection from an activity group | |
| 0–6 mo |
3.77E-04 |
| 6–23 mo |
3.77E-04 |
| 2–4 y old |
3.77E-04 |
| 5–10 y old |
8.81E-04 |
| 11–14 y old |
8.81E-04 |
| 15–18 y old |
8.81E-04 |
| 19–49 y old |
8.81E-04 |
| 50–64 y old |
1.15E-03 |
| 65+ y old |
1.15E-03 |
|
probability of infection from the neighborhood |
|
| 0–6 mo |
8.83E-05 |
| 6–23 mo |
8.83E-05 |
| 2–4 y old |
8.83E-05 |
| 5–10 y old |
2.06E-04 |
| 11–14 y old |
2.06E-04 |
| 15–18 y old |
2.06E-04 |
| 19–49 y old |
2.06E-04 |
| 50–64 y old |
2.70E-04 |
| 65+ y old |
2.70E-04 |
|
probability of infection from the community |
|
| 0–6 mo |
1.91E-07 |
| 6–23 mo |
1.91E-07 |
| 2–4 y old |
1.91E-07 |
| 5–10 y old |
4.47E-07 |
| 11–14 y old |
4.47E-07 |
| 15–18 y old |
4.47E-07 |
| 19–49 y old |
4.47E-07 |
| 50–64 y old |
5.85E-07 |
| 65+ y old |
5.85E-07 |
|
probability of infection in the household |
|
| child to child |
7.40E-02 |
| child to adult |
9.14E-02 |
| adult to child |
9.14E-02 |
| adult to adult | 9.35E-02 |
Contact rates and probability of infection given a contact are aggregated in our model in a set of parameters called probabilities of infection per group. These parameters are estimated for each age-group in activity groups, neighborhood, community, and in households using incidence per age observed in Argentina. More precisely, we used a simplex Nelder-Mead algorithm42 to explore the sets of possible parameters. The quantity to minimize was the sum of squared differences between observed and predicted incidence rates. Additional estimations were performed when influenza incidences were decreased or increased in the sensitivity analysis.
Sources of support
The study was funded by sanofi pasteur.
Disclosure of Potential Conflicts of Interest
N.G., A.G., P.M. and L.L. have received fundings from sanofi pasteur. J.A. and C.R. are employees and P.C. a former employee from the same company.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/18569
References
- 1.Fleming DM, Elliot AJ. Health benefits, risks, and cost-effectiveness of influenza vaccination in children. Pediatr Infect Dis J. 2008;27:S154–8. doi: 10.1097/INF.0b013e31818a5443. [DOI] [PubMed] [Google Scholar]
- 2.Dayan GH, Nguyen VH, Debbag R, Gómez R, Wood SC. Cost-effectiveness of influenza vaccination in high-risk children in Argentina. Vaccine. 2001;19:4204–13. doi: 10.1016/S0264-410X(01)00160-8. [DOI] [PubMed] [Google Scholar]
- 3.Salo H, Kilpi T, Sintonen H, Linna M, Peltola V, Heikkinen T. Cost-effectiveness of influenza vaccination of healthy children. Vaccine. 2006;24:4934–41. doi: 10.1016/j.vaccine.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJ, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA. 2010;303:943–50. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 5.Monto AS, Davenport FM, Napier JA, Francis T., Jr Effect of vaccination of a school-age population upon the course of an A2-Hong Kong influenza epidemic. Bull World Health Organ. 1969;41:537–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Monto AS, Koopman JS, Longini IM., Jr Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121:811–22. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 7.Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weycker D, Edelsberg J, Halloran ME, Longini IM, Jr., Nizam A, Ciuryla V, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 9.Hibbert CL, Piedra PA, McLaurin KK, Vesikari T, Mauskopf J, Mahadevia PJ. Cost-effectiveness of live-attenuated influenza vaccine, trivalent in preventing influenza in young children attending day-care centres. Vaccine. 2007;25:8010–20. doi: 10.1016/j.vaccine.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Elveback LR, Fox JP, Ackerman E, Langworthy A, Boyd M, Gatewood L. An influenza simulation model for immunization studies. Am J Epidemiol. 1976;103:152–65. doi: 10.1093/oxfordjournals.aje.a112213. [DOI] [PubMed] [Google Scholar]
- 11.Halloran ME, Longini IM, Cowart DM, Nizam A. Community interventions and the epidemic prevention potential. Vaccine. 2002;20:3254–62. doi: 10.1016/S0264-410X(02)00316-X. [DOI] [PubMed] [Google Scholar]
- 12.Longini IM, Halloran ME, Nizam A, Wolff M, Mendelman PM, Fast PE, et al. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: implications for influenza epidemic control. Vaccine. 2000;18:1902–9. doi: 10.1016/S0264-410X(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JA, Maher C. Economic Implications of Influenza and Influenza Vaccine. In: Influenza Vaccines for the Future; 2011:425-440. [Google Scholar]
- 14.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 15.Ministerio De Salud De La Nación. Influenza pandémica (H1N1) 2009. República Argentina. 2010.
- 16.Gentile AS De, Ponte Jose M Del. Consenso sobre Actualidad en Vacunas: comité Nacional de Infectología / Updated vaccines consensus. 1998; 96:52-79.
- 17.de Lataillade C, Auvergne S, Delannoy I. 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. J Public Health Policy. 2009;30:83–101. doi: 10.1057/jphp.2008.40. [DOI] [PubMed] [Google Scholar]
- 18.United Nations. World Population Prospects: 2008 Revision Population Database. 2010 estimate. Available at: http://esa.un.org/unpp/p2k0data.asp.
- 19.van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLoS ONE. 2011;6:e17030. doi: 10.1371/journal.pone.0017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wailoo AJ, Sutton AJ, Cooper NJ, Turner DA, Abrams KR, Brennan A, et al. Cost-effectiveness and value of information analyses of neuraminidase inhibitors for the treatment of influenza. Value Health. 2008;11:160–71. doi: 10.1111/j.1524-4733.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 21.Ministerio De Salud De La Nación. Estadisticas Vitales—Informacion Basica Año. 2006.
- 22.INDEC. Censo Nacional de Población, Hogares y Viviendas. 2008. [Google Scholar]
- 23.Grupo Colaborativo de Vigilancia Epidemiológica de la Gripe en Argentina. Argentinean Boletin Epidemiologico 2002–2006. Available at: http://www.grog-argentina.org/grog/front/templates/affich.jsp?siteCode=GROG_LOCAL_ARGENTINA&lang=ES&codeRubrique=11.
- 24.World Health Organisation. Threshold values for intervention cost-effectiveness by Region. 2010. Available at: http://www.who.int/choice/costs/CER_levels/en/index.html.
- 25.Califano G. Vacuna antigripal. In: Sociedad Argentina de Pediatría, Comité Nacional de Infectología, eds. PRIORIDADES PARA LA INCORPORACIÓN DE VACUNAS AL CALENDARIO NACIONAL.; 2011:123-135. [Google Scholar]
- 26.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007:CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Prosser LA, Bridges CB, Uyeki TM, Hinrichsen VL, Meltzer MI, Molinari NA, et al. Health benefits, risks, and cost-effectiveness of influenza vaccination of children. Emerg Infect Dis. 2006;12:1548–58. doi: 10.3201/eid1210.051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess. 2003;7:iii–iv. xi–xiii, 1–170. doi: 10.3310/hta7350. [DOI] [PubMed] [Google Scholar]
- 29.Prosser LA, O’Brien MA, Molinari NA, Hohman KH, Nichol KL, Messonnier ML, et al. Non-traditional settings for influenza vaccination of adults: costs and cost effectiveness. Pharmacoeconomics. 2008;26:163–78. doi: 10.2165/00019053-200826020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ryan J, Zoellner Y, Gradl B, Palache B, Medema J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine. 2006;24:6812–22. doi: 10.1016/j.vaccine.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Rothberg MBB, He S, Rose DNN. Management of influenza symptoms in healthy adults. J Gen Intern Med. 2003;18:808–15. doi: 10.1046/j.1525-1497.2003.20822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augustovski FA, Irazola V, Velasquez A, Gibbons L, Craig B. Argentine valuation of the EQ-5D health states. Value Health. 2009;12:587–96. doi: 10.1111/j.1524-4733.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta-analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatr Infect Dis J. 2007;26:97–106. doi: 10.1097/01.inf.0000253053.01151.bd. [DOI] [PubMed] [Google Scholar]
- 34.Negri E, Colombo C, Giordano L, Groth N, Apolone G, La Vecchia C. Influenza vaccine in healthy children: a meta-analysis. Vaccine. 2005;23:2851–61. doi: 10.1016/j.vaccine.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 35.Villari P, Manzoli L, Boccia A. Methodological quality of studies and patient age as major sources of variation in efficacy estimates of influenza vaccination in healthy adults: a meta-analysis. Vaccine. 2004;22:3475–86. doi: 10.1016/j.vaccine.2004.01.068. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–74. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 37.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–81. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 38.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–6. doi: 10.1016/S0264-410X(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 40.Pan American Health Organisation (PAHO). No Title. 2009. Available at: http://new.paho.org/hq/.
- 41.Giglio ND, Cane AD, Micone P, Gentile A. Cost-effectiveness of the CRM-based 7-valent pneumococcal conjugated vaccine (PCV7) in Argentina. Vaccine. 2010;28:2302–10. doi: 10.1016/j.vaccine.2009.12.070. [DOI] [PubMed] [Google Scholar]
- 42.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes: The Art of Scientific Computing 3rd ed. New York: Cambridge University Press; 2007. [Google Scholar]