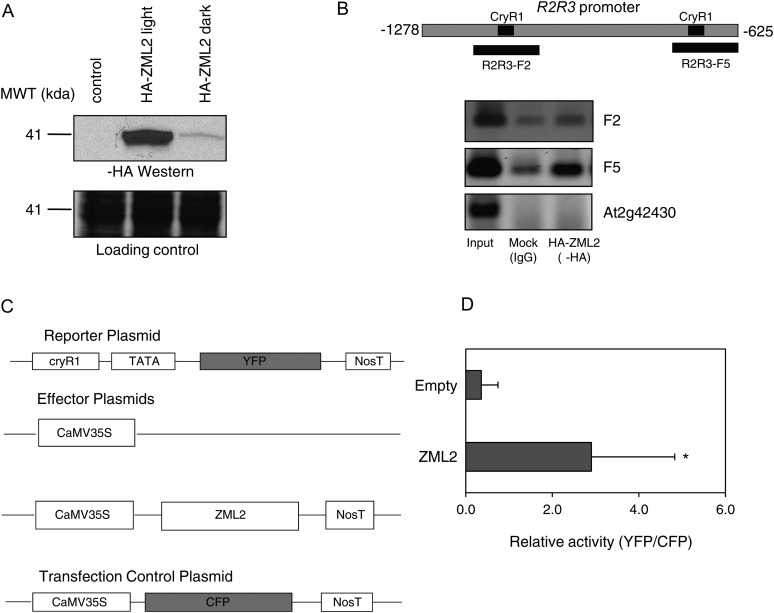
Figure 4.
Binding of ZML2 to CryR1 in Vivo.
(A) Transient expression of HA-ZML2 fusion protein in protoplasts from Arabidopsis cell culture under light (lane 2) and dark (lane 3) conditions. MWT, molecular weight.
(B) ChIP assay using Arabidopsis protoplasts expressing HA-ZML2 fusion protein was performed with anti-HA antibody. The positions of the CryR1 elements identified in the R2R3 promoter and the fragments analyzed by PCR are represented in the scheme. Genomic DNA obtained from ChIP was analyzed by PCR. Immunoprecipitation with IgG antibody was used as a control. Primers for F2 and F5 of the R2R3 promoter and primers for lateral root-specific promoter (At2g42430) were used for PCR amplification. Input, total input chromatin DNA; HA, DNA precipitated using HA antibody; IgG, DNA precipitated using IgG antibody.
(C) Schematic diagram of the effector and reporter constructs used in the transactivation assay. The effector plasmid carried the ZML2 cDNA driven by CaMV 35S promoter. NosT represents the polyadenylation site of nopaline synthetase gene. In the reporter plasmid, the YFP gene was linked to the 218-bp CryR1 fragment and a 35S minimal TATA promoter. As a negative control, an empty effector plasmid was used, and as a control for transfection efficiency, CaMV35S:CFP was cotransfected in each experiment.
(D) CryR1 reporter construct was transfected into protoplasts from Arabidopsis leaves with ZML2 effector construct or 35S empty control vector. To normalize the transfection efficiency, 35S:CFP:NosT was cotransfected in each experiment. Bars represent means of three independent experiments (±sd) with five protoplasts in each experiment. The asterisk indicates significant difference according to the Student’s t test (P < 0.05).