Figure 12.
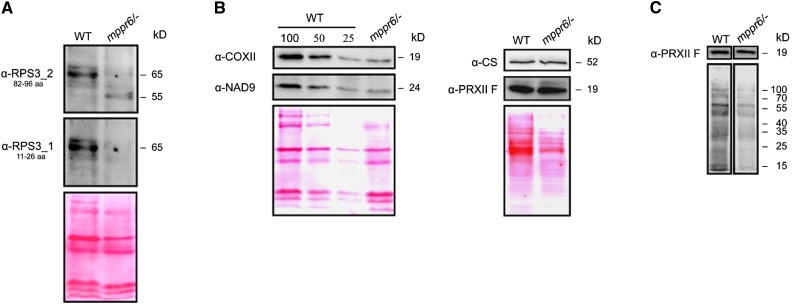
Disruption of MPPR6 Causes Truncation of the RPS3 Protein and a General Translational Deficiency.
(A) Immunodetection of RPS3. Twenty micrograms of mitochondrial protein extracted from wild-type (WT) and mppr6/− callus tissue were separated by SDS-PAGE, blotted on a nitrocellulose membrane, and consecutively probed with affinity purified anti-RPS3 antibodies raised against amino acids 82 to 96 (top panel) or 11 to 26 (middle panel). Ponceau staining of the membrane-bound proteins is shown in the panel below.
(B) Left: Dilutions of membrane protein extracts from wild-type mitochondria were compared with the mppr6/− mutant using antibodies raised against the mitochondria-encoded proteins NAD9 and COXI. 100% corresponds to 20 µg of mitochondrial protein. Right: Immunodetection of the nucleus-encoded mitochondrial proteins PRXII F and CS in soluble extracts from mppr6 mutant and wild-type mitochondrial.
(C) In organello translation of wild-type and mppr6/− mitochondria after inhibition of cytoplasmic translation traces using cycloheximide. The same filter was probed with anti-PRXII F antibody.
[See online article for color version of this figure.]