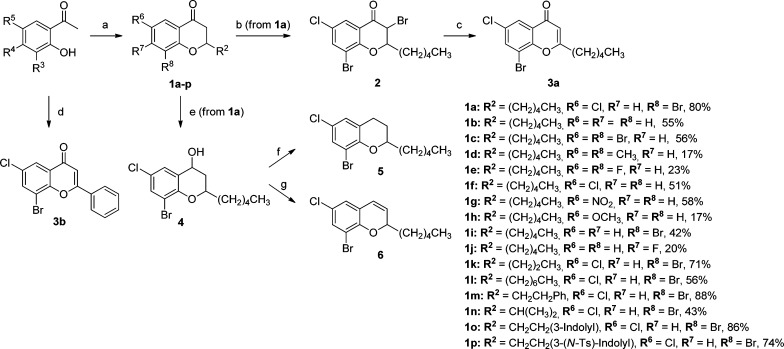
Scheme 1. General Methods for the Syntheses of Compounds 1a–p, 2, 3a,b, and 4–6.
Reagents and conditions: (a) appropriate aldehyde, DIPA, EtOH, MW, 160–170 °C, 1 h, 17–88%; (b) Py·Br3, CH2Cl2, room temp, 2.5 h, 81%, cis/trans ratio 80:20; (c) CaCO3, DMF, MW, 100 °C, 20 min, 84%; (d) i. benzoyl chloride, pyridine, room temp, 2 h; ii. KOH, pyridine, 50 °C, 4 h; iii. HCl, AcOH, reflux, 14 h, 89% (over three steps); (e) NaBH4, MeOH/THF, 0 °C→rt, 15 min, 98%, 95:5 ratio of diastereomers; (f) Et3SiH, BF3·Et2O, CH2Cl2, −78 °C→rt, 19 h, 44%; (g) p-TSA (cat.), MgSO4, toluene, 90 °C, 1.5 h, 63%.