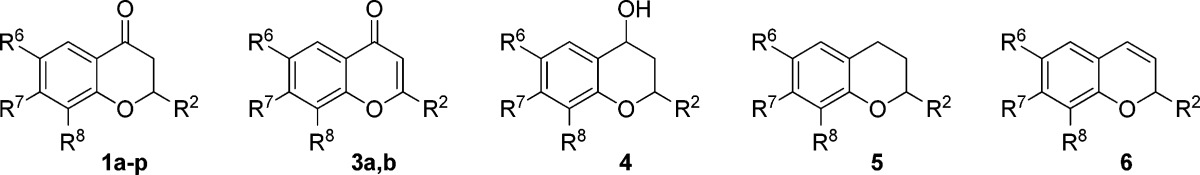
Table 1. Results from Evaluation of Compounds 1a–p, 3a,b, and 4–6 in a SIRT2 Activity Assay.
| compd | R2 | R6 | R7 | R8 | inhibition ± SD at 200 μM (%)a | IC50 (μM)b,c |
|---|---|---|---|---|---|---|
| 1a | (CH2)4CH3 | Cl | H | Br | 88 ± 0.9 | 4.3 (3.5–5.4) |
| (+)-1a | (CH2)4CH3 | Cl | H | Br | 70 ± 0.8 | 4.5 (3.5–5.9) |
| (−)-1a | (CH2)4CH3 | Cl | H | Br | 91 ± 0.8 | 1.5 (1.3–1.7) |
| 1b | (CH2)4CH3 | H | H | H | 4.9 ± 4.8 | n.d. |
| 1c | (CH2)4CH3 | Br | H | Br | 92 ± 1.2 | 1.5 (1.3–1.7) |
| 1d | (CH2)4CH3 | CH3 | H | CH3 | 83 ± 0.7 | 6.2 (4.7–8.1) |
| 1e | (CH2)4CH3 | F | H | F | 30 ± 1.3 | n.d. |
| 1f | (CH2)4CH3 | Cl | H | H | 55 ± 2.4 | n.d. |
| 1g | (CH2)4CH3 | NO2 | H | H | 58 ± 0.7 | n.d. |
| 1h | (CH2)4CH3 | OCH3 | H | H | 20 ± 4.1 | n.d. |
| 1i | (CH2)4CH3 | H | H | Br | 28 ± 1.1 | n.d. |
| 1j | (CH2)4CH3 | H | F | H | 18 ± 1.0 | n.d. |
| 1k | (CH2)2CH3 | Cl | H | Br | 76 ± 1.8 | 10.6 (9.0–12.5) |
| 1l | (CH2)6CH3 | Cl | H | Br | 57 ± 2.5 | n.d. |
| 1m | CH2CH2Ph | Cl | H | Br | 81 ± 0.7 | 6.8 (5.8–8.0) |
| 1n | CH(CH3)2 | Cl | H | Br | 52 ± 1.0 | n.d. |
| 1o | CH2CH2(3-indolyl) | Cl | H | Br | 53 ± 1.7 | n.d. |
| 1p | CH2CH2(N-Ts)(3-indolyl) | Cl | H | Br | 27 ± 1.6 | n.d. |
| 3a | (CH2)4CH3 | Cl | H | Br | 82 ± 0.4 | 5.5 (4.8–6.2) |
| 3b | Ph | Cl | H | Br | 20 ± 1.4 | n.d. |
| 4 | (CH2)4CH3 | Cl | H | Br | 31 ± 3.0 | n.d. |
| 5 | (CH2)4CH3 | Cl | H | Br | 38 ± 1.3 | n.d. |
| 6 | (CH2)4CH3 | Cl | H | Br | 38 ± 1.2 | n.d. |
SD, standard deviation (n = 3).
IC50 (95% confidence interval). IC50 values were determined for compounds showing >70% inhibition of SIRT2 at 200 μM concentration.
n.d. = not determined