Abstract
Colesevelam hydrochloride is a molecularly engineered, second-generation bile acid sequestrant demonstrating enhanced specificity for bile acids which has been approved for use as adjunctive therapy to diet and exercise as monotherapy or in combination with a β-hydroxymethylglutaryl-coenzyme A reductase inhibitor for the reduction of elevated low-density lipoprotein cholesterol in patients with primary hypercholesterolemia. It is also the only lipid-lowering agent currently available in the United States which has been approved for use as adjunctive therapy in patients with type 2 diabetes mellitus whose glycemia remains inadequately controlled on therapy with metformin, sulfonylurea, or insulin. With the recent emphasis upon drug safety by the Food and Drug Administration and various consumer agencies, it is fitting that the role of nonsystemic lipid-lowering therapies such as bile acid sequestrants – with nearly 90 years of in-class, clinically safe experience – should be reexamined. This paper presents information on the major pharmacologic effects of colesevelam, including a discussion of recent data derived from both in vitro and in vivo rodent and human studies, which shed light on the putative mechanisms involved.
Keywords: colesevelam, bile acid sequestrants, cholesterol, low-density lipoprotein
Core evidence outcomes summary for colesevelam hydrochloride in hypercholesterolemia and type 2 diabetes mellitus
| Outcome measure | Evidence | Implications |
|---|---|---|
| Disease-oriented evidence | Lowers LDL-C 16%–18% monotherapy | May be used as monotherapy in mild hypercholesterolemia to reduce LDL-C |
| Increase HDL-C 4%–12%, perhaps by increase in Apo A1 | Considered an inverse risk factor or surrogate marker for CVD | |
| Additive with statins, nicotinic acid, and ezetimibe in further lowering LDL-C | Safe and effective add-on to patients already receiving statin therapy at moderate doses | |
| Lowers FBS, 2-hour PPG, and HbA1c in patients with type 2 DM | Approved as adjunctive therapy for patients with type 2 DM not at goal on metformin, sulfonylureas, or insulin | |
| Patient-oriented evidence | No randomized clinical trial outcome data | First-generation BAS cholestyramine effective as monotherapy in decreasing rate of MI compared to dietary therapy alone |
| Over 10 years clinical experience without serious systemic side effects and no black box warning labels | As a class, >90 years of clinical experience without serious systemic side effects and no black box warning labels | |
| Economic evidence | In patients with poorly controlled DM and not at LDL-C goal, its use as an “add-on” may allow achievement of both HbA1c and LDL-C goals with the use of a single insurance co-pay |
Abbreviations: APO A1, apolipoprotein A1; BAS, bile acid sequestrant; CVD, cardiovascular disease; DM, diabetes mellitus; FBS, fasting blood sugar; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PPG, postprandial glucose.
Introduction
The very first rigorous proof of the lipid hypothesis (ie, lowering the level of cholesterol, a previously known atherosclerotic risk factor, reduces risk for future development of hard clinical cardiovascular morbid events) in man was published over 25 years ago, comparing the use of the bile acid sequestrant (BAS) cholestyramine with placebo in over 3800 patients with hypercholesterolemia who were all partaking in a moderately low cholesterol diet.1 In that study, a modest 13% reduction in low-density lipoprotein cholesterol (LDL-C) by the BAS cholestyramine resulted in a statistically significant 19% decrease in the incidence of nonfatal myocardial infarction. Since that time, numerous other studies have confirmed the lipid hypothesis, utilizing predominantly lipid-lowering agents from another chemical class (β-hydroxymethylglutaryl-coenzyme A [HMGCoA] reductase inhibitors or “statins”). Multiple primary, as well as secondary, prevention trials with these latter agents have demonstrated that even modest reduction of LDL-C from baseline is associated with a significant decrease in cardiovascular disease morbid events, including myocardial infarction and ischemic stroke.2 In addition, some of these trials have demonstrated a relationship between on-treatment LDL-C and the risk of cardiovascular morbid events over a range of LDL-C values.3 Despite the potency of statins, a significant percentage of patients fail to reach the National Cholesterol Education Program goal values, particularly in secondary prevention.4 With regards to the agents themselves, this is in part related to the different shaped dose-response curves of the on-target (LDL-C lowering) and off-target (adverse) effects of the class. Specifically, the dose-response curve of LDL-C-lowering becomes relatively flat early upon upward dose titration, whereas an exponential increase in myalgia, myositis, and transaminasemia is seen with escalating dose. In order, therefore, to achieve the very rigorous LDL-C goals which have been recently promulgated, in addition to statin therapy, it may often be necessary to add a lipid-lowering agent from a different therapeutic class. More will be said about this in the section entitled “Combination lipid-lowering therapy” later in this review.
This paper will discuss the potential role of BAS, in particular the second-generation agent colesevelam, both as monotherapy as well as add-on therapy, in the management of patients with isolated hypercholesterolemia. It will also explore the somewhat serendipitously discovered utility of this agent’s off-target effect upon glucose homeostasis and its subsequent approval as adjunctive therapy in patients with type 2 diabetes mellitus (DM).
The colesevelam molecule
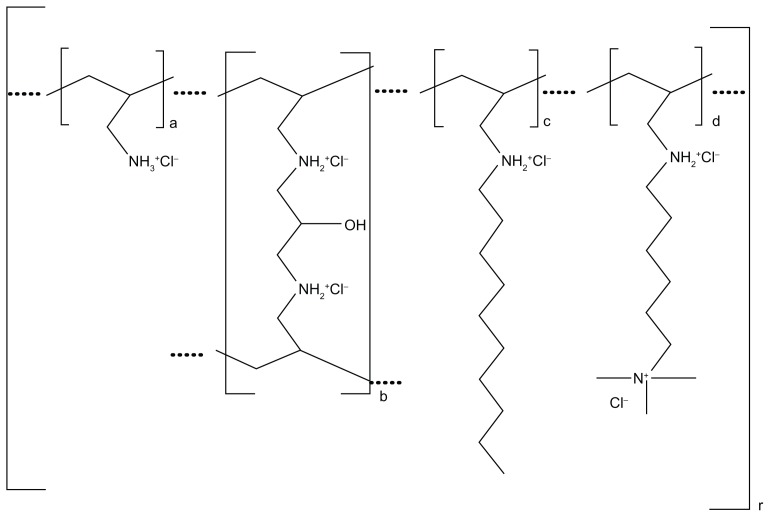
Colesevelam hydrochloride (HCl) is poly(allylamine HCl) crosslinked with epichlorohydrin and alkylated with 1-bromodecane and (6-bromohexyl)-trimethylammonium bromide to form allylamine polymer with 1-chloro-2,3- epoxypropane (6-allylaminohexyl) trimethylammonium chloride and N-allyldecylamine, HCl. The chemical structure is represented in Figure 1 where (a) represents the allyl amine monomer units that have not been alkylated or crosslinked; (b) represents the allyl amine units that have been crosslinked; (c) represents allyl amine units that have been alkylated with a decyl group; and (d) represents allyl amine units that have been alkylated with a 6-trimethylammonium hexyl group, respectively. No regular order of the above groups is implied as crosslinking and alkylation occur randomly along the polymer chains during synthesis. The allyl amine crosslinkage (b) allows the single chain allylamine polymer backbone to chemically bond with other allylamine single chain polymers, creating an extended polymeric network, with the smallest polymer particles having an approximate molecular weight of 1014 Da (100 billion kiloDa).5 As such, unless significantly broken down during its passage in the gut, enteric absorption of the molecule would not be expected. Indeed in rats and dogs, it has been demonstrated, utilizing radiolabeled colesevelam, that neither the allyl amine polymer backbone nor the alkylammonium side chains are absorbed with the amount of radiolabel detected in blood and tissues, as well as recovered in urine, being less than that of the original radiochemical purified preparation after water extraction. This is true for an acute single-dose administration as well as when administered chronically over a 28-day period.5 This virtual total lack of absorption, again within the limits of the radioassay and radiolabel purity (99.96%), was subsequently demonstrated in healthy human volunteers after a single dose of radiolabeled colesevelam.6
Figure 1.
Chemical structure of colesevelam hydrochloride.
All BAS function as resins which are positively charged at intestinal pH (approximately 6.8) binding anionic bile acids in the gut, which is required for their cholesterol-lowering effects. Approximately 90% of commonly administered xenobiotics, however, are also net negatively charged at intestinal pH. First-generation BAS, such as cholestyramine and colestipol, are only charge specific in their binding and therefore also lower the bioavailability of these compounds.7 In vitro binding assays, attempting to simulate intestinal milieu, as well as extensive pharmacokinetic and pharmacodynamic studies in man, have suggested somewhat greater specificity for the second-generation BAS colesevelam. Table 1 reveals the results of pharmacokinetic studies in humans demonstrating the effect upon bioavailability of a number of commonly administered agents when given concomitantly with colesevelam.
Table 1.
Pharmacokinetic colesevelam/drug interactions
| Drug | Cmax | AUC | Notes |
|---|---|---|---|
| Digoxin8 | -- | -- | |
| Ethynilestradiol9 | ↓ | ↓ | 4 hours before OK |
| Fenofibrate10 | -- | -- | Fenofibric acid assay |
| Glyburide9 | ↓ | ↓ | 4 hours before OK |
| Levothyroxine11 | ↓ | ↓ | 4 hours before OK |
| Lovastatin12 | -- | -- | |
| Metoprolol8 | -- | -- | |
| Norenthindrone9 | ↓ | -- | 1 hour before OK |
| Pioglitazone9 | -- | -- | |
| Quinidine8 | -- | -- | |
| Repaglinide9 | ↓ | -- | 1 hour before OK |
| Valproic acid8 | -- | -- | |
| Verapamil8 | ↓ | -- | Cmax ↓ borderline |
| Warfarin8 | -- | -- |
Notes: -- indicates bioavailability of drug unaffected by concomitant administration of colesevelam; ↓ indicates a decrease when given concomitantly with colesevelam relative to the study drug given alone.
Abbreviations: AUC, area under the curve; Cmax, maximum concentration.
Table 2 lists a number of additional agents that are commonly administered with colesevelam for which there is good evidence of additive pharmacodynamic biological effects. It would appear, therefore, that despite the lack of specific pharmacokinetic studies, the bioavailability of these additional agents is also not reduced when administered concomitantly with colesevelam.
Table 2.
Pharmacodynamic additivity
The second-generation BAS colesevelam HCl has been shown to bind bile acids in vitro with a substantially higher affinity than either cholestyramine or colestipol.23 In addition, the proportional increase in fecal bile acids produced by 3.8 g orally administered colesevelam HCl is similar to that observed with 24 g/day of cholestyramine.24,25 As a result, colesevelam HCl is administered at lower doses than first-generation agents,26–28 and has been found to be approximately four times more potent on a weight basis as assessed utilizing pharmacodynamic endpoints such as LDL-C lowering. For those individuals who may have difficulty swallowing tablets, colesevelam (WelChol®) is also available alternatively as a powder which can be mixed with a number of liquids to form a suspension that can be ingested. The affinity, capacity, and kinetics of the molecule’s binding of the sodium salts of glycocholic, glycochenodeoxycholic, and taurodeoxycholic acids are not significantly altered after suspension in water, carbonated water, Coca-Cola®, Sprite®, grape juice, orange juice, tomato juice, or Gatorade®.29 Based upon the above, Food and Drug Administration (FDA) approval was eventually granted for a citrus-like flavored powder preparation of colesevelam which can be mixed with water (approval October 2009) or a variety of other beverages (approval July 2011).
Clinical use in lipid-lowering monotherapy
Colesevelam LDL-C reduction – clinical
In July 2000, colesevelam HCl received FDA approval for use as adjunctive therapy to diet and exercise for the reduction of elevated LDL-C in patients with primary hypercholesterolemia (Fredrickson type IIa hyperlipoproteinemia).28 The results of at least four randomized, double-blind, placebo-controlled, parallel designed trials led to that approval. Two of these trials seemed to suggest a log-linear relationship between the placebo-corrected resultant decrease in LDL-C concentration and the dose of colesevelam, suggesting perhaps that a certain threshold might be needed to achieve clinical efficacy.30,31 The largest trial, however, did not confirm this over a large dosing range from 2.3–4.5 g.32 The fourth trial compared the efficacy of once-daily dosing with 3.8 g versus twice-daily dosing with 1.9 g and found both regimens equally effective in lowering LDL-C.33 Combining the results of the four studies in over 600 actively treated patients, placebo-corrected, weighted average LDL-C reductions of 2.2%, 8.0%, 11.1%, and 16.6% were observed for doses of approximately 1.5 g, 2.3 g, 3.0 g, and 3.8 g of colesevelam HCl respectively. In a similar manner, serum apolipoprotein B (Apo B) concentration was shown to decrease in a dose-dependent manner.32 Consistent with this latter finding, colesevelam has been shown to decrease LDL particle number by 6.8% and 13.7% at doses of 3.0 and 3.8 g, respectively, accompanied by a small, but statistically significant increase of 1.1% in LDL particle size.34
The total size of the bile acid pool remains unaltered after cholecystectomy,35,36 although alterations in the production of individual primary and secondary bile acids have been reported.35,37 After cholecystectomy, the enterohepatic cycling of bile acids is enhanced in the fasting state,37 as only the small intestine remains as a physical pump regulating these dynamics. Total fecal bile acids, moreover, are increased three-to ten-fold in patients with postcholecystectomy diarrhea. 38 The package insert recommends that colesevelam be taken with or shortly after meals, ie, postprandially. While bile salts are released from the gallbladder postprandially, the author knows of no study – prospective or retrospective – examining the relative efficacy of LDL-C lowering by colesevelam in patients who are stable postcholecystectomy versus those with intact functioning gallbladders.
Colesevelam LDL-C reduction – putative mechanism
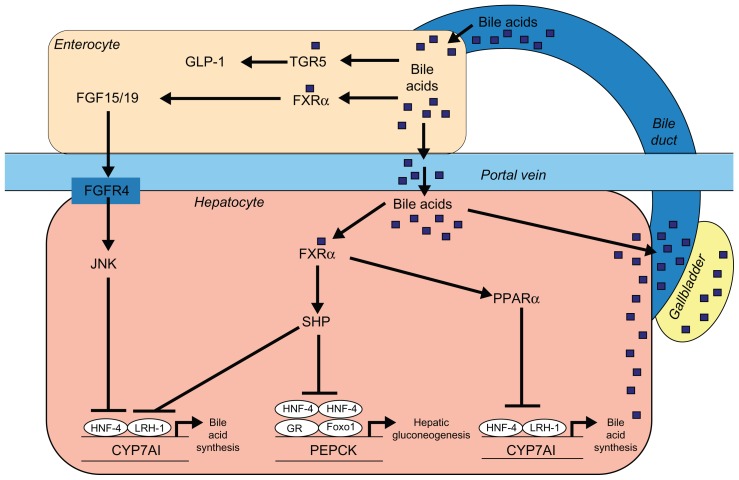
By interrupting the return of bile acid to the liver, BAS prevent the activation of the nuclear transcription factor farnesoid X receptor (FXR). In the liver, this inhibition leads to a decrease in the induction of small heterodimer partner-1, with removal of the inhibitory effect of this moiety upon liver receptor homolog-1, a coactivator of transcription factor liver X receptor-α (LXRα), which in turn results in upregulation of the rate-limiting enzyme cholesterol 7 α-hydroxylase (Cyp7A1) responsible for bile acid synthesis from cholesterol.39 By preventing binding of bile acids to FXR of small intestinal epithelial cells, there is decreased generation of fibroblast growth factor-19 (FGF19) and decreased transport via the portal circulation of that moiety to the liver, with decreased binding, therefore, of FGF19 to FGF liver receptor-4, resulting in decreased activation of c-Jun N-terminal kinase and increased Cyp7A1 enzymatic activity,39 possibly through upregulation of hepatic nuclear factor-4α (Figure 2).40 With activation of Cyp7A1, there is increased hepatocyte utilization of cholesterol with less availability of substrate for oxysterol formation and, therefore, less activation of LXR. With decreased hepatocyte concentration of free cholesterol, the sterol regulatory element-binding protein-2 (SREBP-2)/SREBP cleavage activating protein (SCAP) complex migrates from the endoplasmic reticulum to the Golgi apparatus where SREBP-2 is subjected to staged proteolysis by site-1 protease (S1P) and site-2 protease (S2P), the resulting N-terminal motif migrating to the cell nucleus where it binds to sterol response elements resulting in upregulation of hepatocyte LDL receptor proteins as well as HMGCoA reductase. With production of additional LDL receptors, there is increased internalization of LDL-C by the liver with subsequent reduction of serum LDL-C. Upregulation of HMGCoA reductase, the rate-limiting enzyme controlling cholesterol synthesis may, however, be an important limiting factor with regards to the efficacy of BAS monotherapy for the reduction of LDL-cholesterol.
Figure 2.
Bile acids as hormones in the regulation of metabolic processes.
Reproduced with permission from Reasner.83
Abbreviations: CYP7A1, cholesterol 7 α-hydroxylase; FGF15/19, fibroblast growth factor 15/19; FGFR4, fibroblast growth factor receptor-4; Foxo1, forkhead box protein O1; FXRα, farnesoid X receptor-α; GLP-1, glucagon-like peptide-1; GR, glucocorticoid receptor; HNF-4, hepatocyte nuclear factor-4; JNK, c-Jun N-terminal kinase; LRH-1, liver receptor homolog-1; PEPCK, phosphoenolpyruvate carboxykinase; PPARα, peroxisome proliferator-activated receptor-α; SHP, small heterodimer partner.
Colesevelam HDL-C increase – clinical
In two of the three studies described above,30,31 placebocorrected HDL-C increased significantly by 7.2%–12.2% at doses of colesevelam exceeding 3.0 g/day. In the larger, longer follow-up study of Insull et al,32 HDL-C increased significantly in a nondose-dependent manner by 4% with doses of colesevelam varying between 2.3–4.5 g. In the latter study, as well as in two additional placebo-controlled trials,22,41 Apo A1 also increased significantly by 3%–8% in a nondose-related manner.
Colesevelam HDL-C increase – putative mechanism
In human Caco-2 adenocarcinoma cells, it has been demonstrated that by decreasing exposure to bile acids ( physiologically similar to interrupting return of bile acids to the liver by colesevelam), there is less activation of FXR, less induction of small heterodimer partner-1, less inhibition of LXRα by the latter and thereby upregulation of the gene encoding adenosine triphosphate-binding cassette transporter protein A1, thus enhancing reverse cholesterol transport and thereby increasing HDL-C.42 In hepatoblastoma HepG2 cells, decreasing exposure to bile acids results in decreased activity of a negative FXR response element at the “C-site” of the promoter gene for Apo A1, resulting in induction of Apo A1.43
Colesevelam triglycerides – clinical
With regards to placebo-corrected change in serum triglycerides, there has been wide variation in the reported results of three clinical trials in patients with type IIa hyperlipoproteinemia referenced above. All, however, reported no statistically significant increase in serum triglycerides. In one study, serum triglycerides increased by 2.5%–23.2%.31 In a second study, the data and calculations presented in Table 2, while somewhat difficult to interpret, appear to suggest an increase in triglycerides of 13.2% and 18.8% at 3.0 g and 3.8 g colesevelam, respectively.30 In the largest single study, triglycerides increased in a nondose-dependent manner from 0%–5%.32 These latter results are quite consistent with the 2.8%–5.4% placebo-corrected increase in serum triglycerides reported previously with the use of cholestyramine in a much larger cohort of patients (n ≈ 1900) with very similar baseline lipid values.1 The apparent absence of a statistically significant triglyceride elevation reported in the above colesevelam studies may reflect inadequate patient sample size in the individual studies and/or the large inherent biological variability of triglycerides. It is the author’s experience that the absolute and perhaps relative increase in serum triglycerides seen in patients treated with colesevelam may be greater in patients with insulin resistance and/or a greater degree of hypertrig-lyceridemia at baseline. Consistent with this hypothesis is the 14% (P < 0.004) and 17% (P = 0.02) increase in median serum triglyceride level that has been reported in subjects with prediabetes44 and metabolic syndrome,45 with baseline triglycerides of 175 mg/dL and 193 mg/dL, respectively, treated with colesevelam therapy. In this regard, it must be noted that colesevelam has neither been rigorously investigated as monotherapy in patients with mixed hyperlipidemia, nor is FDA approved for use in that setting.
Colesevelam triglycerides – putative mechanism
Interrupting the return of bile acids to the liver results in decreased ligand binding to FXR and decreased activation of small heterodimer partner-1 – an inhibitor of liver receptor homolog-1 and LXRα, which results in relative activation of the latter transcription factors. This in turn results in the induction of SREBP-1c (the “zipper transcription factor”),46 glucose- or insulin-activated movement of the SREBP-1c/SCAP complex from the endoplasmic reticulum to the Golgi apparatus, and staged proteolysis by S1P and S2P. The resulting N-terminal motif migrates to the cell nucleus where there is binding to sterol response elements resulting in the upregulation of acetyl-CoA synthetase, acetyl-CoA carboxylase, and fatty acid synthase – enzymes involved in the synthesis of fatty acids, ultimately resulting in increased triglyceride synthesis. By preventing binding of bile acids to FXR of small intestinal epithelial cells, there is decreased generation of FGF19, decreased transport via the portal circulation of that moiety to the liver, decreased activity of signal transducer and activator of transcription-3, an inhibitor of SREBP-1c expression, and thereby increased activity of SREBP-1c by a mechanism possibly independent of the hepatocyte FGF liver receptor-4 (Figure 2 – latter steps not shown).47 In addition to these anabolic alterations, there may also be decreased catabolism of triglycerides. In human hepatoma HepG2 cells, decreased exposure to bile acids results in decreased activation of a positive FXR element on the promoter gene of peroxisome proliferator-activated receptor-α, lesser induction of Apo C2 (lipoprotein lipase activator), and greater induction of Apo C3 (lipoprotein lipase inhibitor), thereby resulting in decreased lipoprotein lipase activity.48
Colesevelam high-sensitivity C-reactive protein – clinical
With regards to inflammatory markers, as monotherapy, colesevelam 3.8 g/day has been shown to decrease high-sensitivity C-reactive protein by 18.7% in a small randomized, double-blind, placebo-controlled study.49
Clinical use in combination lipid-lowering therapy
Use with statin therapy – clinical
Due to overwhelmingly positive outcome studies in patients treated for hypercholesterolemia with HMGCoA reductase inhibitors (statins), these agents remain the backbone of lipid-lowering therapy. With every statin that has been investigated, the use of colesevelam HCl as add-on therapy for further management of hypercholesterolemia has demonstrated further reduction in LDL-C,13,14,21,22,41,50 suggesting a class effect interaction in this regard. In two parallel design studies which utilized high dose 3.8 g/day colesevelam as add-on therapy,14,22 the weighted average additional LDL-C reduction from baseline compared to statin monotherapy was 14%. Three studies, one each with atorvastatin,14 simvastatin,22 and lovastatin,41 had a relatively ideal study design and potentially shed light on the pharmacodynamic interaction. Assume the use of two cholesterol-lowering drugs “A” and “B” as monotherapy is associated with percentage LDL-C reductions of “X” and “Y,” respectively, from baseline. A modification of the formula of Schectman and Hiatt, {100 - [100 + (X*Y/100) - X - Y]/100} where X*Y represents the simple product of X and Y, defines the percentage of LDL-C reduction achieved by concomitant use of “A” and “B” if their effects are purely additive.51 Measured reductions of LDL-C greater than predicted would be consistent with synergy, whereas reductions less than predicted would imply mechanistic antagonism. Using this approach, the difference between measured and predicted LDL-C reduction, expressed as a percentage of predicted reduction, was 1.2%,14 10.8%,41 and 14.9%22 in the above three studies with the simple weighted patient average being 11.1%. In a similar manner, the difference between measured and predicted Apo B reduction, expressed as a percentage of predicted reduction, was 2.6%14 and 21.4%22 with the simple weighted patient average being 15.1%. These results are most consistent with pharmacologic additivity, while a small degree of synergy cannot be excluded.
In a pooled analysis of three trials, colesevelam HCl, when added to background statin therapy, further lowered high-sensitivity C-reactive protein by 23% in comparison to statin monotherapy alone.13
Use with statin therapy – putative mechanism
In contrast to using two or more drugs from the same therapeutic class, the combined use of drugs from different therapeutic classes, working by different mechanisms of action on the same endpoint (in this case LDL-C lowering), will in general demonstrate at least pharmacodynamic additivity. Exceptions of course include synergy, where the measured benefit exceeds that which would be predicted by additivity alone, and antagonism where the mechanism of action of one agent presumably interferes in some manner with the mechanism of action of the second agent. With inhibition by statins of HMGCoA reductase, the rate-limiting enzyme in cholesterol biosynthesis, there is a decrease in the cytoplasmic concentration of cholesterol. As discussed earlier, with decreased hepatocyte concentration of cholesterol, through a complex mechanism involving SREBP2, SCAP, S1P, S2P, and perhaps other proteins, there is a “compensatory” upregulation of gene expression for HMGCoA reductase and LDL-receptor proteins, the latter allowing for increased clearance of LDL-C from the circulation. Decreased intracellular cytoplasmic cholesterol concentration, however, also results in less direct enzyme feedback inhibition as well as less enzyme degradation by polyubiquitination – both effects mediated via cholesterol binding to a sterol sensing domain of the HMGCoA reductase enzyme itself. These latter two effects along with the upregulation of HMGCoA reductase described above, limit the utility of serum cholesterol lowering with statin monotherapy. BAS lower hepatocyte cytoplasmic concentration of cholesterol differently by upregulating Cyp7A1 and thereby increasing synthesis of bile acid from cholesterol as previously described. The increased utilization of cholesterol for bile acid synthesis engendered by BAS, coupled with the continued strong inhibition of the rate-limiting enzyme in cholesterol biosynthesis by statins, produces an additive effect upon LDL-receptor protein synthesis and a further lowering of serum cholesterol.
Use with ezetimibe therapy – clinical
Five studies have examined the combined use of ezetimibe and colesevelam HCl. In all of these, the dose of ezetimibe was 10 mg. Two of these were computerized chart reviews; in one case the second agent had been added to the first (n = 16),18 while in the other ezetimibe had been added to colesevelam 3.1 g average dose (n = 33).17 In the former case, the addition of the second agent resulted in a 23% (P < 0.05), and in the latter case a 19% (P < 0.05), further reduction in LDL-C compared with monotherapy alone. A third small (n = 12), prospective, open label, randomized, nonplacebo-controlled, add-on study reported on patients randomized initially to either ezetimibe or colesevelam 3.8 g, with the alternative agent added 6 weeks later. After an additional 6 weeks on double drug therapy, a 39% decrease in LDL-C relative to baseline was measured which represented a further average 20% decrease (P < 0.005) in LDL-C in comparison to either monotherapy.15 Employing the modification of the formula of Schectman and Hiatt described previously,51 the difference between measured and predicted LDL-C reduction, expressed as a percentage of predicted, approached zero at −6.2%, probably consistent with pharmacologic additivity. The remaining two trials were randomized, double-blind, placebo-controlled, add-on studies where colesevelam 3.8 g versus placebo had been added to patients treated with ezetimibe. In one trial (n = 86),19 colesevelam produced a further 14.5% reduction in LDL-C at 6 weeks (P < 0.0001). In the second trial (n = 18),52 only a further 6% reduction in LDL-C was reported at both 6 weeks and 12 weeks of combination therapy (P = 0.102). A type II error secondary to inadequate sample size (only nine subjects actually received active dual therapy) might explain the latter result.
The above pharmacodynamic data, while not as robust as in the case of statin/colesevelam combination therapy does suggest possible pharmacodynamic additivity when these two intestinally active agents are used together. While use of the ezetimibe/colesevelam combination is not FDA approved, physicians have employed this therapy off- label in patients who are statin intolerant.
Use with ezetimibe therapy – putative mechanism
While both ezetimibe and colesevelam HCl are intestinally active agents, their mechanism of action is quite distinct. Ezetimibe, and more importantly its active metabolite ezetimibe glucuronide, binds to the Niemann-Pick C1-like-1 receptor at the jejunal epithelial cell brush border, preventing formation of the clathrin-coated vesicles which allow endocytosis of the cholesterol Niemann-Pick C1-like-1 receptor complex.53 With decreased intestinal cholesterol absorption, there is decreased cholesterol esterification by intestinal acetyl-CoA acetyltransferase-2, resulting in hepatic uptake of chylomicron remnants with decreased cholesterol content, resulting ultimately in both decreased production of very LDL-C (VLDL-C) particles and decreased hepatocyte free cholesterol concentration.54 The mechanism whereby colesevelam lowers hepatocyte cholesterol concentration has been previously described and is unrelated to the above. The decrease in free cholesterol concentration within the hepatocyte, through a complex mechanism involving SREBP2, SCAP, S1P, S2P, and perhaps other proteins, results in the upregulation of gene expression for HMGCoA reductase and LDL receptor proteins, the latter allowing increased clearance of LDL-C from the circulation.
Use with nicotinic acid therapy – clinical
A previous trial employing the BAS colestipol (30 g/day administered in two divided doses) and immediate-release nicotinic acid (4.3 g/day) revealed a placebo-corrected decrease in LDL-C of 38% compared to baseline.55 This value exceeds the decrease in LDL-C that has been reported in patients receiving monotherapy with either agent.56,57 A single parallel design, placebo-controlled study revealed a further 17% reduction of LDL-C when colesevelam HCl 3.8 g was added to patients already receiving atorvastatin (mean dose 20 mg) and extended-release nicotinic acid (Niaspan®) 2000 mg/day.20 Enrollment has been completed for the randomized, double-blind, placebo-controlled CERTAIN (Colesevelam Treatment for Impaired Fasting Glucose During Niacin Therapy; NCT01239004) phase IV clinical trial, which will report the effects of colesevelam upon serum lipids as well as markers of glycemia in patients with dyslipidemia and impaired fasting glucose who are being treated with up to 2000 mg of extended-release nicotinic acid. Currently, the combination of colesevelam HCl and nicotinic acid is not FDA approved for the management of dyslipidemia.
Use with nicotinic acid therapy – putative mechanism
The proposed mechanisms of action of nicotinic acid with regards to lipid modulation are complex. With regards to its LDL-C-lowering effects, binding to the G protein-coupled receptor-109A of adipocytes results in inhibition of hormone sensitive lipase with decreased free fatty acid release into the circulation. This coupled with direct inhibition of the enzyme diacylglycerol O-acyltransferase-2 results in a decrease in triglyceride synthesis, resulting in accelerated hepatic Apo B degradation, resulting in decreased release of VLDL particles into the circulation.58 As already discussed, the mechanism of BAS to lower LDL-C is independent of the above, and, therefore, pharmacodynamic additivity might be expected.
Use with fibrate therapy – clinical
While there are no reports of the use of gemfibrozil with colesevelam, there are reports of the use of gemfibrozil with the first-generation BAS cholestyramine and colestipol.59,60 In these studies, there was further reduction of LDL-C relative to monotherapy with gemfibrozil, while the triglyceride lowering effect of the fibrate did not appear significantly impaired. A single, randomized, double-blind, placebo-controlled, add-on study examined the effect of adding 3.8 g/day colesevelam HCl versus placebo to patients with mixed hyperlipidemia being treated with fenofibrate 160 mg/day.61 The addition of colesevelam resulted in a 12.4% decrease in LDL-C relative to those patients treated with fenofibrate alone. Also, the addition of colesevelam to fenofibrate therapy further reduced the levels of non-HDL-C and Apo B without statistically affecting the triglyceride-lowering effects of fenofibrate. These results are consistent with the reported lack of pharmacokinetic interactive effects when these drugs are administered together.10 While the above studies suggest a potential use for the combination of fibrates with colesevelam in patients with mixed hyperlipidemia, their use together has not been FDA approved.
Use with fibrate therapy – putative mechanism
Fibrates as agonists of the peroxisome proliferator-activated receptor-α nuclear transcription factor cause a decrease in triglyceride synthesis, resulting in decreased release by the liver of large VLDL1 and VLDL2 triglyceride rich VLDL particles. With peroxisome proliferator-activated receptor α-mediated upregulation of lipoprotein lipase, there is also increased catabolism of triglyceride rich lipoproteins VLDL and IDL,62 further resulting in triglyceride relatively deficient LDL particles which are poor substrates for both hepatic lipase as well as cholesterol ester transfer protein.63 The latter enzyme, moreover, may also be downregulated directly by fibrate therapy.64 The result is a decrease in total LDL particle number with a relative increase in the number of large buoyant LDL particles and a relative decrease in small dense LDL particles.65 All fibrates appear to decrease transcription of Cyp7A1, resulting in decreased bile acid synthesis,66,67 increased hepatocyte cholesterol concentration, and potentially resulting in a downregulation of LDL receptor protein. The latter, however, does not appear to be significant, perhaps related to fibrate-mediated upregulation of the adenosine triphosphate-binding cassette subfamily G member-5 gene coding for sterolin-1, resulting in increased secretion of cholesterol into bile, lowering hepatocellular content of free cholesterol.66 This explains the formation of supersaturated bile and gallstones with fibrate use. Consistent with this explanation, the addition of cholestyramine, with its upregulating effects upon Cyp7A1, to gemfibrozil in patients with familial combined hyperlipidemia has been shown to decrease the cholesterol saturation of bile.59 Fibrates do, however, differ in the degree of LDL-C reduction at approved doses, and this may be due to additional mechanisms such as selective increase in SREBP-2 transcription by bezafibrate.66
Use with phytosterol (phytostanol) therapy – clinical
A metaanalysis of 41 trials employing an average dose of 2 g of stanol (saturated double bond at C5–C6 of corresponding sterol) or sterol esters has revealed an average 10% reduction in LDL-C when used as monotherapy.68 Both stanol and sterol esters decrease LDL-C equally. Phytostanols, however, are much more poorly absorbed with blood levels less than 10% that of phytosterols, and this may have significance with regards to their respective effects upon endothelial function.69 A small open-label, add-on study of eleven patients has previously suggested a further 38% decrease in LDL-C when cholestyramine 8 g daily was added to patients already consuming 2.3 g of stanol ester margarine and 20 mg simvastatin daily.70 A randomized, double-blind, placebo-controlled study of 55 patients treated with statin and 3.8 g colesevelam HCl examined the addition of 1 g plant sterol-fortified orange juice versus placebo orange juice taken twice daily and found no additional LDL-C-lowering effect.71 Of note, orange juice and colesevelam were ingested together at the same times.
Use with phytosterol (phytostanol) therapy – putative mechanism
Phytosterols, being more hydrophobic than cholesterol, are thought to displace cholesterol from micelles and on that basis decrease cholesterol absorption by the intestine. As colesevelam lowers LDL-C by a completely different mechanism (see “Colesevelam LDL-C reduction – putative mechanism” above), it is conceivable that either the study was underpowered (only 27 patients received sterol ester-fortified juice) to detect a small anticipated difference such as 10% further LDL-C lowering, or colesevelam may have bound the plant sterol, removing it from the gut and effectively reducing its availability to the micelle.
Adjunctive therapy for type 2 DM
Colesevelam effects upon blood sugar and hemoglobin A1c (HbA1c)
The GLOWS (Glucose-Lowering Effect of WelChol) study was a prospective, double-blind, placebo-controlled, parallel design, add-on trial in which 65 less than optimally controlled type 2 DM patients (mean HbA1c 7.9%; mean fasting blood sugar [FBS] 170 mg/dL; mean 1-hour postprandial glucose 269 mg/dL), being treated with sulfonylurea and/or metformin, were randomized to receive either colesevelam 3.8 g/day or placebo. Treatment with BAS resulted in a least squares mean difference of −0.5% in HbA1c (P = 0.007), −14 mg/dL (−8.2%; P = 0.12) in FBS, and −32 mg/dL (−11.9%; P = 0.026) in postprandial glucose.72 A second larger study of similar design reported on 287 patients with type 2 DM (mean HbA1c 8.3%; mean FBS 165 mg/dL), slightly more than one-third of whom were being treated with insulin therapy alone with the remainder receiving insulin therapy in combination with oral antidiabetes agents. At week 16, treatment with colesevelam 3.8 g/day resulted in a least squares mean difference of −0.5% (P < 0.001) in HbA1c and −16 mg/dL (−9.5%; P = 0.03) in FBS.73 A third study of similar design reported on 316 patients with type 2 DM (mean HbA1c 8.1%; mean FBS 178 mg/dL) who were being treated with metformin, 86% and 43% of whom were also being treated with sulfonylureas and thiazolidinediones, respectively. At week 26, treatment with colesevelam 3.8 g/day resulted in a least squares mean difference of −0.5% (P < 0.001) in HbA1c and −14 mg/dL (−7.9%; P = 0.01) in FBS.74 In this latter study, there was also no change in fasting insulin level or insulin sensitivity. Additional studies with similar design have been performed in patients with prediabetes (mean HbA1c 6.0%; FBS 110–125 mg/dL [median 103 mg/dL] and/or 2-hour postoral glucose tolerance test 140–199 mg/dL [mean 154 mg/dL])44 and metabolic syndrome (mean HbA1c 5.85%; mean FBS 108 mg/dL; mean 2-hour oral postprandial glucose 182 mg/dL).45 In the former study, treatment with colesevelam resulted in least squares mean and median differences in HbA1c and FBS of −0.1% (P = 0.02) and −2.0 mg/dL (−1.9%; P = 0.02), respectively.44 There was no significant change in least squares mean of 2-hour postoral glucose tolerance test, median fasting insulin, or 2-hour postoral glucose tolerance test insulin. In the latter study, the mean FBS decreased 5 mg/dL (4.7%; P < 0.02), while HbA1c, fasting insulin, and insulin sensitivity remained unchanged with colesevelam therapy.45
In the above five clinical studies, there was a high correlation (Pearson’s r = 0.97) between the change in FBS (in mg/dL) and the level of baseline FBS (in mg/L). There was likewise a high correlation (Pearson’s r = 0.92) between the percentage reduction in FBS and the baseline level of FBS. These findings suggest that both the absolute, as well as relative decrease in FBS affected by colesevelam, may be dependent upon the baseline degree of glycemia. This finding is also consistent with the greater absolute decrease in HbA1c seen in patients with baseline HbA1c > 8.0% versus those with HbA1c ≤ 8.0% reported in some of the individual studies.72,73 Of note, in all the above studies, the anticipated decrease in LDL-C, based upon the previously discussed mechanism of action of BAS, was also demonstrated.
With the exception of a single study which utilized colestipol,75 studies utilizing colestimide76,77 and cholestyramine78 have also documented a decrease in FBS and/or HbA1c relative to placebo in patients with type 2 DM, strongly suggesting these actions are a class effect of BAS.
Colesevelam effects upon blood sugar and HbA1c – putative mechanism of action
In the past few years, using rodent models of DM/obesity, and more recently elegant metabolic studies performed in man, an attempt has been made to better elucidate the mechanisms responsible for the glycemic effects described above. Possibly due to the number of transgenic animal models examined and the potential differences in comparison to type 2 DM in man, the literature appears at times to be contradictory. A detailed discussion of the multiple hypotheses generated by this work is beyond the scope of this review and the reader is referred to a number of excellent monographs.79–83 A brief summary of some of the more recent hypothesis-generating data, only, will be presented here.
Certain rodent models suggest that BAS acting as “FXR antagonists” and perhaps, therefore, upregulators of LXR, are involved in the regulation of glucose metabolism,84–86 resulting in improved insulin sensitivity.87 This effect upon insulin sensitivity has never, however, been conclusively demonstrated in man. Peripheral insulin resistance as measured by the hyperinsulinemic euglycemic clamp method appears unaltered during colesevelam administration.88–90 On the other hand, hepatic insulin sensitivity, as perhaps reflected by the Matsuda index,91 may be improved with colesevelam treatment,90 but this has not always been confirmed. 88 While FGF19 plasma levels do fall and cholic acid synthesis does increase during colesevelam therapy, consistent with a lesser activation of intestinal FXR, there is apparently no correlation between markers of insulin resistance/glucose metabolism and bile acid metabolism.89,92 Gluconeogenesis does not appear to be affected, although an effect upon glycogenolysis cannot be excluded.89 Studies in man appear consistent in excluding an effect upon glucose absorption when colesevelam is given.88,89 Fasting insulin and glucagon levels remain unchanged after colesevelam therapy.89,90
Fasting and postprandial glucagon-like peptide-1 levels are increased in man with colestimide93 and colesevelam treatment,89 and this result is consistent with animal experiments.87,94 BAS, by binding bile acids in the intestine, may prevent optimal micellar solubilization of fatty acids, diminishing their normally efficient and nearly complete absorption by the jejunum. These fatty acids are now free to pass into the ileum, where they may enter intestinal “L-cells” and via G protein-coupled receptors 40 and 120 stimulate incretin secretion.95 Free fatty acids, moreover, are a known stimulator of cholecystokinin (CCK) synthesis by the enterochromaffin mucosal “I-cells” of the proximal small intestine.96 CCK binding to CCK-1 receptors on the basolateral membrane of ileal L-cells can also evoke postprandial glucagon-like peptide-1 release.96 CCK, moreover, through a cholinergic mechanism, appears to evoke release of pancreatic lipase, potentially increasing further the level of intestinal free fatty acids,97 that as mentioned above cannot be adequately solubilized. CCK, by delaying gastric emptying, may also independently further ameliorate postprandial hyperglycemia.98 A recent study, in patients with impaired fasting glucose, has indeed demonstrated an increase in CCK plasma levels in colesevelam- treated patients after an oral meal but not after intravenous glucose administration,99 seemingly consistent with the above mechanism and consistent with other clinical studies in type 2 DM patients that have demonstrated an improvement in postprandial glycolytic disposal.72,89,90 Recently, an intestinal G protein-coupled bile acid receptor TGR5, by enhancing oxidative phosphorylation and increasing the intracellular ratio of adenosine triphosphate/diphosphate, has also been demonstrated to affect a release of postprandial glucagon-like peptide-1.100 While colesevelam use is associated with an increase in fecal bile acid excretion,24 it is unclear whether bile acid bound to the polymer would be biologically active at the TGR5 receptor.
Safety and tolerability
Table 3 reveals adverse reactions that have been reported in at least 2% of patients, and more commonly than placebo, during clinical trials. In such trials, colesevelam has been employed in patients with estimated glomerular filtration rate as low as 30 mL/minute/1.73 m2 without difficulty with regards to safety or efficacy. While BAS are contraindicated in patients with complete biliary obstruction, in which bile is not secreted into the intestine, there are otherwise no special considerations in patients with hepatic impairment. Pooled analyses of clinical studies has revealed a small, but statistically significant, increase in the level of liver transaminase as well as alkaline phosphatase, which have, however, remained within the normal reference range.101 As the medication on a weight basis is approximately four times more potent than cholestyramine, less resin bulk in the intestine results in a reduction of bothersome gastrointestinal side effects of constipation, flatulence, and bloating in comparison with firstgeneration agents.1,28,102 While no study has directly compared colesevelam with the older BAS, in the Lipid Research Clinics Coronary Primary Prevention Trial, 39% of patients taking cholestyramine reported moderate-to-severe constipation in the first year of study,1 a figure far exceeding that reported in clinical trials with colesevelam. During postmarketing surveillance, bowel obstruction with cholestyramine, while extremely rare, has been reported.103–105 This may be related to the relative impotence of this first-generation BAS and the resulting bulk of cholestyramine resin (8–24 g) needed to achieve a hypocholesterolemic effect. Use of colesevelam of course is contraindicated in patients with a history of bowel obstruction. Pooled analyses from studies with a relatively small number of patients have revealed a small, but statistically significant, decrease in the blood levels of fat soluble vitamins, which have, however, remained within the normal reference range.101 This is probably related to less than optimal solubilization of vitamins A, D, E, and K through interference with micelle formation in the absence of intestinal free bile acids. It is currently recommended that individuals taking supplements of the above vitamins take them at least 4 hours before or after taking colesevelam.106 The potential for other drug interactions has been discussed earlier. The oral suspension formulation of colesevelam described previously does contain 48 mg of phenylalanine per 3.75 g packet of colesevelam and should be avoided in patients with known phenylketonuria. While clinical experience is quite limited, colesevelam is the only lipid-lowering agent given a pregnancy category “B” rating by the FDA, with first-generation BAS cholestyramine and colestid granules, fibrates, nicotinic acid, and ezetimibe rated “C” and statins rated “X.” The reader is referred to the colesevelam (WelChol®, Parsippany, NJ) manufacturer’s package insert106 for a comprehensive review of product use recommendations.
Table 3.
Adverse effects of colesevelam hydrochloride
| Adverse reaction | Colesevelam (%) | Placebo (%) | Notes |
|---|---|---|---|
| Accidental injury | 3.7 | 2.7 | |
| Asthenia | 3.6 | 1.9 | |
| Constipation | 11.0 | 7.0 | |
| Dyspepsia | 8.3 | 3.5 | |
| Nausea | 4.2 | 3.9 | |
| Hypoglycemia | 3.0 | 2.3 | Adjunctive Rx in DM |
| Myalgia | 2.1 | 0.4 | |
| Pharyngitis | 3.2 | 1.9 | |
| Rhinitis | 3.2 | 3.1 |
Abbreviations: DM, diabetes mellitus; Rx, prescription.
As colesevelam is not appreciably absorbed (see previous section “The colesevelam molecule”), discussion of systemic toxicology related directly to the molecule itself or metabolites is not germane. While originally perceived as merely biological detergents aiding in the solubilization and absorption of dietary fats, over the past decade it has been demonstrated that bile acids, through their known agonist activity at the nuclear FXR transcription factor, act as hormones involved in multiple different physiological systems as previously described in this manuscript. While the pharmacodynamic activity of BAS is ultimately dependent upon their ability to bind and remove bile acids from the intestinal tract, these “nonsystemic” pharmacologic preparations, by decreasing the availability of bile acids at their receptors, have the potential to have both multiple beneficial, as well as deleterious, systemic effects. Examples of the former would include the lowering of LDL-C, increase in HDL-C, and decrease in FBS, postprandial sugar, and HbA1c. An example of the latter might be the triglyceride elevation associated with their use in certain patients. Indeed, the use of colesevelam is contraindicated in patients with serum triglycerides in excess of 500 mg/dL and should be used only with caution in patients with serum triglycerides between 300–500 mg/dL.106 While additional pernicious effects are always possible, it is gratifying to know that after nearly 90 years of clinical use of this therapeutic class, there has never been a “black box” label warning issued, or any evidence of increased malignancy or other serious “off-target” systemic effects related to the use of BAS.
Conclusion
Statins, first available in the United States 25 years ago, remain the backbone of lipid-lowering therapy due to their potency as well as proven efficacy in decreasing morbid cardiovascular disease events. Extensive use of this class of agents over the past two decades has revealed, however, early peaking, and then relatively flat dose-response curves for “on-target” LDL-C-lowering effects. Indeed, the different efficacies of the various statins in lowering LDL-C can be attributed to differences in the degree of LDL-C lowering seen at their lowest approved FDA dose. On the other hand, the dose-response curves for “off-target” effects such as myopathy and transaminasemia exhibit an exponential type of increase with escalating drug dose. Recent data obtained in both primary107 and secondary108 prevention settings, have also revealed a dose related increase in new onset type 2 DM in patients on chronic statin therapy. In addition, postmarketing surveillance has confirmed the rare side effect of mild cognitive decline in statin users, which had been previously suggested.109,110
With this as background, the more aggressive lipid-lowering goals that have been recently promulgated, particularly in the secondary prevention arena,111,112 require LDL-C reduction in many cases in excess of 50% – a result achievable only with potent statins and often with high doses of those agents. The concept of combination lipid-lowering therapy has, therefore, been reborn in an attempt to reach LDL-C goal, minimize “off-target” statin effects, and optimize patient compliance. Pharmacologically, this is an attempt to remain on the steep portion of the “on-target” pharmacodynamic dose-response curve of two active agents of different therapeutic classes, thereby maximizing efficacy and minimizing bothersome dose sensitive “off-target” side effects.
With statins remaining the backbone of lipid-lowering therapy, the question of what constitutes the “ideal” add-on lipid-lowering agent must be raised. Ideally, the agent should demonstrate pharmacodynamic additivity or synergy in lowering LDL-C compared to statin monotherapy. Its use, when combined with statin therapy, should have demonstrated significant reduction in “hard” cardiovascular disease endpoints relative to statin monotherapy in long-term clinical studies. It should be free of systemic side effects, free of significant drug–drug and drug–food interactions, and be safe and well tolerated by all patient groups, including diabetics as well as those with chronic kidney and liver disease. While such an agent does not exist today, the long-term safety record of BAS, the efficacy of at least a first-generation agent to reduce clinical myocardial infarction as monotherapy,1 and the use of agents such as colesevelam to potentially off-set the development of DM in patients receiving statin therapy, make it a good time to remember the “forgotten” BAS.82
Footnotes
Disclosure
Dr Zema is a member of the National Speakers Bureau for Daichii Sankyo, manufacturer of WelChol® (colesevelam).
References
- 1.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment; prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Moye LA, Davis BR, et al. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation. 1998;97(15):1446–1452. doi: 10.1161/01.cir.97.15.1446. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation Project Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556–563. doi: 10.1016/j.amjcard.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum DP, Petersen JS, Ducharme S, Markham P, Goldberg DI. Absorption, distribution and excretion of GT31–104, a novel bile acid sequestrant, in rats and dogs after acute and subchronic administration. J Pharm Sci. 1997;86(5):591–595. doi: 10.1021/js9603820. [DOI] [PubMed] [Google Scholar]
- 6.Heller DP, Burke SK, Davidson DM, Donovan JM. Absorption of colesevelam hydrochloride in healthy volunteers. Ann Pharmacother. 2002;36(3):398–403. doi: 10.1345/aph.1A143. [DOI] [PubMed] [Google Scholar]
- 7.Bays HE, Dujovne CA. Drug interactions of lipid-altering drugs. Drug Saf. 1998;19(5):355–371. doi: 10.2165/00002018-199819050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Donovan JM, Stypinski D, Stiles MR, Olson TA, Burke SK. Drug interactions with colesevelam hydrochloride, a novel, potent lipid-lowering agent. Cardiovasc Drugs Ther. 2000;14(6):681–690. doi: 10.1023/a:1007831418308. [DOI] [PubMed] [Google Scholar]
- 9.Brown KS, Armstrong IC, Wang A, et al. Effect of the bile acid sequestrant colesevelam on the pharmacokinetics of pioglitazone, repaglinide, estrogen estradiol, norethindrone, levothyroxine, and glyburide. J Clin Pharmacol. 2010;50(5):554–565. doi: 10.1177/0091270009349378. [DOI] [PubMed] [Google Scholar]
- 10.Jones MR, Baker BA, Mathew P. Effect of colesevelam HCl on single-dose fenofibrate pharmacokinetics. Clin Pharmacokinet. 2004;43(13):943–950. doi: 10.2165/00003088-200443130-00006. [DOI] [PubMed] [Google Scholar]
- 11.Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19(1):77–79. doi: 10.1089/thy.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan JM, Kisicki JC, Stiles MR, Tracewell WG, Burke SK. Effect of colesevelam on lovastatin pharmacokinetics. Ann Pharmacother. 2002;36(3):392–397. doi: 10.1345/aph.1A144. [DOI] [PubMed] [Google Scholar]
- 13.Bays HE, Davidson M, Jones MR, Abby SL. Effects of colesevelam hydrochloride on low-density lipoprotein cholesterol and high-sensitivity C-reactive protein when added to statins in patients with hypercholesterolemia. Am J Cardiol. 2006;97(8):1198–1205. doi: 10.1016/j.amjcard.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake D, Insull W, Jr, Toth P, Davidson D, Donovan JM, Burke SK. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis. 2001;158(2):407–416. doi: 10.1016/s0021-9150(01)00437-3. [DOI] [PubMed] [Google Scholar]
- 15.Zema MJ. Colesevelam HCl and ezetimibe combination therapy provides effective lipid-lowering in difficult-to-treat patients with hypercholesterolemia. Am J Ther. 2005;12(4):306–310. doi: 10.1097/01.mjt.0000155109.69831.a3. [DOI] [PubMed] [Google Scholar]
- 16.Zema MJ. Add-on therapy for hypercholesterolemia: a pilot comparison of two gastrointestinally-acting agents in statin-treated patients. J Clin Lipidol. 2009;3(2):119–124. doi: 10.1016/j.jacl.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Xydakis AM, Guyton JR, Chiou P, Stein JL, Jones PH, Ballantyne CM. Effectiveness and tolerability of ezetimibe add-on therapy to a bile acid resin-based regimen for hypercholesterolemia. Am J Cardiol. 2004;94(6):795–797. doi: 10.1016/j.amjcard.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Rivers SM, Kane MP, Busch RS, Bakst G, Hamilton RA. Colesevelam hydrochloride-ezetimibe combination lipid-lowering therapy in patients with diabetes or metabolic syndrome and a history of statin intolerance. Endocr Pract. 2007;13(1):11–16. doi: 10.4158/EP.13.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Bays H, Rhyne J, Abby S, Lai YL, Jones M. Lipid-lowering effects of colesevelam HCl in combination with ezetimibe. Curr Med Res Opin. 2006;22(11):2191–2200. doi: 10.1185/030079906X148436. [DOI] [PubMed] [Google Scholar]
- 20.Moore A, Phan BA, Challender C, Williamson J, Marcovina S, Zhao XQ. Effects of adding extended-release niacin and colesevelam to statin therapy on lipid levels in subjects with atherosclerotic disease. J Clin Lipidol. 2007;1(6):620–625. doi: 10.1016/j.jacl.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Kawashiri M, Nohara A, Noguchi T, et al. Efficacy and safety of coadministration of rosuvastatin, ezetimibe and colestimide in heterozygous familial hypercholesterolemia. Am J Cardiol. 2012;109(3):364–369. doi: 10.1016/j.amjcard.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Knapp HH, Schrott H, Ma P, et al. Efficacy and safety of combination simvastatin and colesevelam in patients with primary hypercholesterolemia. Am J Med. 2001;110(5):352–360. doi: 10.1016/s0002-9343(01)00638-6. [DOI] [PubMed] [Google Scholar]
- 23.Braunlin W, Zhorov E, Smisek D, et al. In vitro comparison of bile acid binding to colesevelam HCl and other bile acid sequestrants. Polymer Prepr. 2000;41(1):708–709. [Google Scholar]
- 24.Donovan JM, Von Bergmann K, Setchell KD, et al. Effects of colesevelam HCl on sterol and bile acid excretion in patients with type IIa hypercholesterolemia. Dig Dis Sci. 2005;50(7):1232–1238. doi: 10.1007/s10620-005-2765-8. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Ahrens EH, Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971;78(1):94–121. [PubMed] [Google Scholar]
- 26.Colestid® (colestipol) [prescribing information] Kalamazoo, MI: Pharmacia and Upjohn; 2003. [Google Scholar]
- 27.Questran® (cholestyramine) powder [prescribing information] Princeton, NJ: Bristol-Myers Squibb; 1997. [Google Scholar]
- 28.WelChol® (colesevelam) [prescribing information] Parsippany, NJ: Daiichi Sankyo; 2000. [Google Scholar]
- 29.Hanus M, Zhorov E. Bile acid salt binding with colesevelam HCl is not affected by suspension in common beverages. J Pharm Sci. 2006;95(12):2751–2759. doi: 10.1002/jps.20734. [DOI] [PubMed] [Google Scholar]
- 30.Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159(16):1893–1900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 31.Mesner CH, Burke SK, Dillon M. Cholestagel for the treatment of hypercholesterolemia. Paper presented at: 13th International Symposium on Drugs Affecting Lipid Metabolism; May 30, 1998–June 3; Florence Italy. [Google Scholar]
- 32.Insull W, Jr, Toth P, Mullican W, et al. Effectiveness of colesevelam hydrochloride in decreasing LDL cholesterol in patients with primary hypercholesterolemia: a 24-week randomized controlled trial. Mayo Clin Proc. 2001;76(10):971–982. doi: 10.4065/76.10.971. [DOI] [PubMed] [Google Scholar]
- 33.Ose L, MacMahon OL, Theisen K, Farnoer M, Davidson D, Burke S. Once per day and split dosing of colesevelam in patients with type IIa hypercholesterolemia. Paper presented at: 5th International Symposium on Multiple Risk Factors in Cardiovascular Disease: Global Assessment and Intervention; October 28–31, 1999; Venice, Italy. [Google Scholar]
- 34.Rosenson RS. Colesevelam HCl reduces LDL particle number and increases LDL size in hypercholesterolemia. Atherosclerosis. 2006;185(2):327–330. doi: 10.1016/j.atherosclerosis.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Roda E, Aldini R, Mazzella G, et al. Enterohepatic circulation of bile acids after cholecystectomy. Gut. 1978;19(7):640–649. doi: 10.1136/gut.19.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kullak-Ublick GA, Paumgartner G, Berr F. Long-term effects of cholecystectomy on bile-acid metabolism. Hepatology. 1995;21(1):41–45. doi: 10.1002/hep.1840210109. [DOI] [PubMed] [Google Scholar]
- 37.Berr F, Stellaard F, Pratschke E, Paumgartner G. Effects of cholecystectomy on the kinetics of primary and secondary bile acids. J Clin Invest. 1989;83(5):1541–1550. doi: 10.1172/JCI114050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arlow FL, Dekovich AA, Priest RJ, Beher WT. Bile acid-mediated postcholecystectomy diarrhea. Arch Intern Med. 1987;147(7):1327–1329. [PubMed] [Google Scholar]
- 39.Out C, Hageman J, Bloks VW, et al. Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration. Hepatology. 2011;53(6):2075–2085. doi: 10.1002/hep.24286. [DOI] [PubMed] [Google Scholar]
- 40.Inoue Y, Yu AM, Yim SH, et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4-alpha. J Lipid Res. 2006;47(1):215–227. doi: 10.1194/jlr.M500430-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson MH, Toth P, Weiss S, et al. Low-dose combination therapy with colesevelam hydrochloride and lovastatin effectively decreases low-density lipoprotein cholesterol in patients with primary hypercholesterolemia. Clin Cardiol. 2001;24(6):467–474. doi: 10.1002/clc.4960240610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol. 2002;16(9):2065–2076. doi: 10.1210/me.2001-0194. [DOI] [PubMed] [Google Scholar]
- 43.Claudel T, Sturm E, Duez H, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR reponse element. J Clin Invest. 2002;109(7):961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Handelsman Y, Goldberg RB, Garvey WT, et al. Colesevelam hydrochloride to treat hypercholesterolemia and improve glycemia in prediabetes: a randomized, prospective study. Endocr Pract. 2010;16(4):617–628. doi: 10.4158/EP10129.OR. [DOI] [PubMed] [Google Scholar]
- 45.Vega GL, Dunn FL, Grundy SM. Effect of colesevelam hydrochloride on glycemia and insulin sensitivity in men with the metabolic syndrome. Am J Cardiol. 2011;108(8):1129–1135. doi: 10.1016/j.amjcard.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 46.DeBose-Boyd RA, Ou J, Goldstein JL, Brown MS. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci U S A. 2001;98(4):1477–1482. doi: 10.1073/pnas.98.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284(15):10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 49.Devaraj S, Autret B, Jialal I. Effects of colesevelam hydrochloride (WelChol) on biomarkers of inflammation in patients with mild hypercholesterolemia. Am J Cardiol. 2006;98(5):641–643. doi: 10.1016/j.amjcard.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Huijgen R, Abbink EJ, Bruckert E, et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, doubleblind, controlled trial. Clin Ther. 2010;32(4):615–625. doi: 10.1016/j.clinthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Schectman G, Hiatt J. Dose-response characteristics of cholesterol-lowering drug therapies: implications for treatment. Ann Intern Med. 1996;125(12):990–1000. doi: 10.7326/0003-4819-125-12-199612150-00011. [DOI] [PubMed] [Google Scholar]
- 52.Knopp RH, Tsunehara C, Retzlaff BM, et al. Lipoprotein effects of combined ezetimibe and colesevelam hydrochloride versus ezetimibe alone in hypercholesterolemic subjects: a pilot study. Metabolism. 2006;55(12):1697–1703. doi: 10.1016/j.metabol.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Ge L, Wang L, Qi W, et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7(6):508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14(3):99–108. doi: 10.5551/jat.14.99. [DOI] [PubMed] [Google Scholar]
- 55.Blankenhorn D, Nessim SA, Johnson RL, Sammarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233–3240. [PubMed] [Google Scholar]
- 56.Gaw A, Packard CJ, Lindsay GM, et al. Effects of colestipol alone and in combination with simvastatin on apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1996;16(2):236–249. doi: 10.1161/01.atv.16.2.236. [DOI] [PubMed] [Google Scholar]
- 57.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. New Engl J Med. 2009;361(22):2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 58.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Odman B, Ericsson J, Lindmark M, Berglund L, Angelin B. Gemfibrozil in familial combined hyperlipidaemia: effect of added low-dose cholestyramine on plasma and biliary lipids. Eur J Clin Invest. 1991;21(3):344–349. doi: 10.1111/j.1365-2362.1991.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 60.Houlston R, Quiney J, Watts GF, Lewis B. Gemfibrozil in the treatment of resistant familial hypercholesterolaemia and type III hyperlipoproteinaemia. J R Soc Med. 1988;81(5):274–276. doi: 10.1177/014107688808100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenney J, Jones M, Abby S. Safety and efficacy of colesevelam hydrochloride in combination with fenofibrate for the treatment of mixed hyperlipidemia. Curr Med Res Opin. 2005;21(9):1403–1412. doi: 10.1185/030079905x59157. [DOI] [PubMed] [Google Scholar]
- 62.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leiteisdorf E, Fruehart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 63.Guerin M, Dolphin PJ, Chapman MJ. A new in vitro method for the simultaneous evaluation of cholesterol ester exchange and mass transfer between HDL and apoB-containing lipoprotein subspecies. Identification of preferential cholesterol ester acceptors in human plasma. Arterioscler Thromb. 1994;14(2):199–206. doi: 10.1161/01.atv.14.2.199. [DOI] [PubMed] [Google Scholar]
- 64.van der Hoogt CC, de Haan W, Westerterp M, et al. Fenofibrate increases HDL-cholesterol by reducing cholesteryl ester transfer protein expression. J Lipid Res. 2007;48(8):1763–1771. doi: 10.1194/jlr.M700108-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Chan SY, Mancini GB, Ignaszewski A, Frohlich J. Statins but not fibrates improve the atherogenic to anti-atherogenic lipoprotein particle ratio: a randomized crossover study. BMC Clin Pharmacol. 2008;8:10. doi: 10.1186/1472-6904-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roglans N, Vazquez-Carrera M, Alegret M, et al. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol. 2004;59(12):855–861. doi: 10.1007/s00228-003-0704-1. [DOI] [PubMed] [Google Scholar]
- 67.Stahlberg D, Reihner E, Rudling M, Berglund L, Einarsson K, Angelin B. Influence of bezafibrate on hepatic cholesterol metabolism in gallstone patients: reduced activity of cholesterol 7 alpha-hydroxylase. Hepatology. 1995;21(4):1025–1030. doi: 10.1002/hep.1840210421. [DOI] [PubMed] [Google Scholar]
- 68.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78(8):965–978. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 69.Hallikainen M, Lyyra-Laitinen T, Laitinen T, et al. Endothelial function in hypercholesterolemic subjects: effects of plant stanol and sterol esters. Atherosclerosis. 2006;188(2):425–432. doi: 10.1016/j.atherosclerosis.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Gylling H, Miettinen TA. LDL cholesterol lowering by bile acid malabsorption during inhibited synthesis and absorption of cholesterol in hypercholesterolemic coronary subjects. Nutr Metab Cardiovasc Dis. 2002;12(1):19–23. [PubMed] [Google Scholar]
- 71.Linnebur SA, Capell WH, Saseen JJ, Wolfe P, Eckel RH. Plant sterols added to combination statin and colesevelam hydrochloride therapy failed to lower low-density lipoprotein cholesterol concentrations. J Clin Lipidol. 2007;1(6):626–633. doi: 10.1016/j.jacl.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29(1):74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168(14):1531–1540. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 74.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168(18):1975–1983. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 75.Bandisode MS, Boshell BR. Hypocholesterolemic activity of colestipol in diabetes. Curr Ther Res Clin Exp. 1975;18(2):276–284. [PubMed] [Google Scholar]
- 76.Suzuki T, Oba K, Futamia S, et al. Blood glucose-lowering activity of colestimide in patients with type 2 diabetes and hypercholesterolemia: a case-control study comparing colestimide with acarbose. J Nihon Med Sch. 2006;73(5):277–284. doi: 10.1272/jnms.73.277. [DOI] [PubMed] [Google Scholar]
- 77.Yamakawa T, Takano T, Utsunomiya H, Kadonosono K, Okamura A. Effect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemia. Endocr J. 2007;54(1):53–58. doi: 10.1507/endocrj.k05-098. [DOI] [PubMed] [Google Scholar]
- 78.Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med. 1994;121(6):416–422. doi: 10.7326/0003-4819-121-6-199409150-00004. [DOI] [PubMed] [Google Scholar]
- 79.Davidson MH. Interrupting bile-acid handling and lipid and glucose control: effects of colesevelam on glucose levels. J Clin Lipidol. 2008;2(2):S29–S33. doi: 10.1016/j.jacl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Staels B. A review of bile acid sequestrants: potential mechanism(s) for glucose-lowering effects in type 2 diabetes mellitus. Postgrad Med. 2009;121(3 Suppl 1):25–30. doi: 10.3810/pgm.2009.05.suppl53.290. [DOI] [PubMed] [Google Scholar]
- 81.Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32(Suppl 2):S237–S245. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bays HE, Goldberg RB. The “forgotten” bile acid sequestrants: is now a good time to remember? Am J Ther. 2007;14(6):567–580. doi: 10.1097/MJT.0b013e31815a69fc. [DOI] [PubMed] [Google Scholar]
- 83.Reasner CA. Reducing cardiovascular complications of type 2 diabetes by targeting multiple risk factors. J Cardiovasc Pharmacol. 2008;52(2):136–144. doi: 10.1097/FJC.0b013e31817ffe5a. [DOI] [PubMed] [Google Scholar]
- 84.Laffitte BA, Chao LC, Li J, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100(9):5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim TH, Kim H, Park JM, et al. Interrelationship between liver X receptor alpha, sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma, and small heterodimer partner in the transcriptional regulation of glucokinase gene expression in liver. J Biol Chem. 2009;284(22):15071–15083. doi: 10.1074/jbc.M109.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Commerford SR, Vargas L, Dorfman SE, et al. Dissection of the insulin-sensitizing effects of liver X receptor ligands. Mol Endocrinol. 2007;21(12):3002–3012. doi: 10.1210/me.2007-0156. [DOI] [PubMed] [Google Scholar]
- 87.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G419–G424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 88.Henry RR, Aroda VR, Mudaliar S, Garvey WT, Chou HS, Jones MR. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(1):40–46. doi: 10.1111/j.1463-1326.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55(2):432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz SL, Lai YL, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8(2):179–188. doi: 10.1089/met.2009.0049. [DOI] [PubMed] [Google Scholar]
- 91.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 92.Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52(4):1455–1464. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki T, Oba K, Igari Y, et al. Colestimide lowers plasma glucose levels and increases plasma glucagon-like peptide-1 (7–36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J Nihon Med Sch. 2007;74(5):338–343. doi: 10.1272/jnms.74.338. [DOI] [PubMed] [Google Scholar]
- 94.Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2010;334(1):164–170. doi: 10.1124/jpet.110.166892. [DOI] [PubMed] [Google Scholar]
- 95.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beglinger S, Drewe J, Schirra J, Goke B, D’Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like peptide-1 secretion. J Clin Endocrinol Metab. 2010;95(2):879–886. doi: 10.1210/jc.2009-1062. [DOI] [PubMed] [Google Scholar]
- 97.Soudah HC, Lu Y, Hasler WL, Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol. 1992;263(1 Pt 1):G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- 98.Liddle RA, Rushakoff RJ, Morita ET, Beccaria L, Carter JD, Goldfine ID. Physiological role for cholecystokinin in reducing postprandial hyperglycemia in humans. J Clin Invest. 1988;81(6):1675–1681. doi: 10.1172/JCI113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marina AL, Utzschneider KM, Wright LA, Montgomery BK, Marcovina SM, Kahn SE. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care. 2012;35(5):1119–1125. doi: 10.2337/dc11-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melian EB, Plosker GL. Colesevelam. Am J Cardiovasc Drugs. 2001;1(2):141–146. doi: 10.2165/00129784-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 102.Andrade SE, Walker AM, Gottlieb LK, et al. Discontinuation of anti-hyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332(17):1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 103.Lloyd-Still JD. Cholestyramine therapy and intestinal obstruction in infants. Pediatrics. 1977;59(4):626–627. [PubMed] [Google Scholar]
- 104.Merten DF, Grossman H. Intestinal obstruction associated with cholestyramine therapy. AJR Am J Roentgenol. 1980;134(4):827–828. doi: 10.2214/ajr.134.4.827. [DOI] [PubMed] [Google Scholar]
- 105.Tonstad S, Knudtzon J, Sivertsen M, Refsum H, Ose L. Efficacy and safety of cholestyramine in peripubertal and prepubertal children with familial hypercholesterolemia. J Pediatr. 1996;129(1):42–49. doi: 10.1016/s0022-3476(96)70188-9. [DOI] [PubMed] [Google Scholar]
- 106.WelChol® (colesevelam) [prescribing information] Parsippany, NJ: Daiichi Sankyo; updated 2008. [Google Scholar]
- 107.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 108.Coleman CI, Reinhart K, Kluger J, White CM. The effects of statins on the development of new-onset type 2 diabetes: a metaanalysis of randomized controlled trials. Curr Med Res Opin. 2008;24(5):1359–1362. doi: 10.1185/030079908x292029. [DOI] [PubMed] [Google Scholar]
- 109.Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108(7):538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 110.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117(11):823–829. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 111.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 112.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113(19):2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]