Abstract
Background
Comparative analysis, which aims at investigating ecological and evolutionary patterns among species, may help at targeting reservoirs of zoonotic diseases particularly in countries presenting high biodiversity. Here, we developed a simple method to target rodent reservoirs using published studies screening microparasite infections.
Methods
We compiled surveys of microparasites investigated in rodents trapped in Thailand. The data comprise a total of 17,358 rodents from 18 species that have been investigated for a total of 10 microparasites (viruses, bacteria and protozoans). We used residual variation of microparasite richness controlled for both rodent sample size and pathogens’ screening effort to identify major rodent reservoirs and potential risky habitats.
Results
Microparasite species richness was positively related to rodent sample size and pathogens’ screening effort. The investigation of the residual variations of microparasite species richness showed that several rodent species harboured more pathogens than expected by the regression model. Similarly, higher pathogen richness than expected was observed in rodents living in non-flooded lands, forests and paddy fields.
Conclusion
Our results suggest to target some rodent species that are not commonly investigated for pathogen screening or surveillance such as R. adamanensis or B. savilei, and that non-flooded lands and forests should be more taken into caution, whereas much surveys focused on paddy rice fields and households.
Keywords: microparasite richness, rodents, Southeast Asia, zoonosis, habitat, prioritization
Targeting reservoirs of zoonotic diseases is a challenge particularly in countries presenting high biodiversity. Comparative analysis, which aims at investigating ecological and evolutionary patterns among species, may help at indentifying wildlife reservoirs using published studies.
Introduction
We aim at presenting a simple way to prioritize reservoir species in relation to their capacity to carry multiple agents of potential diseases. We illustrate this simple method with rodent-borne diseases in Thailand, a country that showed major outbreaks of several rodent-borne diseases in the past years (1). We focused on rodents as carriers, vectors or reservoirs of numerous zoonotic diseases, notably microparasites (2). Thailand presents the advantage to harbour a rich biodiversity, although at threat (3), and to have been quite intensively surveyed for rodent-borne diseases. Using published surveys investigating rodent-borne diseases in rodents in this country we aim at identifying the major rodent species reservoirs, at least in term of their capacity to host multiple pathogens. For this, we first investigate the relationship between the pathogen species richness observed among rodent species and the screening effort and, second, we use the residual variations of this relationship to prioritize rodents and evaluate the potential risky habitats.
Materials and methods
We compiled surveys of microparasites investigated in rodents trapped in Thailand (from 27 references given in supplementary appendix). The data comprise a total of 17,358 rodents from 18 species of murine rodents that have been investigated for a total of 10 microparasites (Table 1). The microparasites were viruses, bacteria and protozoans. Viruses were: Hantaviruses, Lymphocytic choriomeningitis virus (family Arenaviridae, genus Arenavirus), Rabies virus and Hepatitis E virus. These viruses are directly transmitted between rodent hosts and are all major pathogens of humans. Three bacteria were investigated: Leptopsira spp. agents of leptospirosis, Bartonella spp. agents of bartonellosis and Orientia tsutsugamushi, the agent of scrub typhus. Bartonella sp. and Orientia tsutsugamushi are arthropod-borne agents, whereas Leptospira spp. are indirectly transmitted via contact with water or soils contaminated by urine of infected rodents. From the three protozoans, only Toxoplasma gondii can infect humans whereas Trypanosoma spp. and Babesia spp. infect livestock and more rarely humans. Altogether 1,716 rodents (10%) have been found positive for at least one pathogen. Microparasite richness was defined as the number of pathogen species for which each rodent species was found positive.
Table 1.
Survey of infection by microparasites (viruses, bacteria, protozoans) of rodent species in Thailand, with number of positive individuals and number of investigated individuals between brackets (see references in supplementary materials)
| Species | Leptospira spp. | Orientia spp. | Bartonella spp. | Hanta virus | Herpes virus | LCM virus | Rabies virus | Toxoplasma gondii | Trypanosoma spp. | Babesia spp. |
|---|---|---|---|---|---|---|---|---|---|---|
| Bandicota indica | 102 (1006) | 101 (755) | 12 (167) | 60 (932) | 3 (164) | 20 (166) | 0 (276) | 1 (37) | 11 (192) | 17 (30) |
| Bandicota savilei | 12 (464) | 52 (189) | 2 (33) | 3 (197) | 2 (12) | 5 (14) | 0 (17) | 0 (11) | 13 (64) | |
| Berylmys berdmorei | 0 (6) | 2 (9) | 5 (20) | 0 (12) | 0 (3) | 0 (4) | 1 (23) | |||
| Berylmys bowersi | 0 (9) | 0 (5) | 0 (5) | 0 (3) | ||||||
| Leopoldamys edwardsi | 0 (23) | 2 (16) | 4 (10) | |||||||
| Maxomys surifer | 0 (19) | 8 (33) | 0 (6) | 1 (150) | 2 (38) | 6 (22) | ||||
| Mus caroli | 0 (6) | 0 (69) | 0 (5) | 0 (67) | 0 (6) | 0 (3) | ||||
| Mus cervicolor | 0 (12) | 0 (17) | 1 (2) | 0 (85) | 0 (1) | 0 (13) | ||||
| Mus musculus | 0 (4) | 0 (2) | 0 (3) | |||||||
| Mus cookii | 0 (40) | 0 (1) | 0 (17) | |||||||
| Niviventer fulvescens | 1 (4) | 0 (8) | 0 (10) | 0 (11) | 0 (13) | 0 (2) | ||||
| Rattus andamanensis | 1 (12) | 0 (1) | 0 (7) | 1 (3) | ||||||
| Rattus argentiventer | 6 (102) | 5 (23) | 0 (107) | 15 (31) | 1 (9) | 4 (62) | ||||
| Rattus exulans | 48 (1242) | 20 (465) | 1 (71) | 24 (667) | 0 (3) | 3 (47) | 0 (1) | 1 (79) | 22 (266) | 0 (17) |
| Rattus losea | 6 (86) | 82 (638) | 2 (24) | 1 (119) | 0 (10) | 0 (2) | 0 (4) | 0 (1) | 3 (12) | |
| Rattus norvegicus | 179 (860) | 11 (36) | 26 (309) | 22 (48) | 17 (54) | 0 (1) | 0 (34) | 1 (14) | ||
| Rattus tanezumi | 107 (1858) | 542 (2284) | 7 (73) | 21 (900) | 5 (74) | 3 (69) | 0 (139) | 11 (256) | 7 (143) | |
| Rattus tiomanicus | 0 (97) | 26 (105) | 0 (2) |
We used information on main habitats of rodents following Jittapalapong et al. (4, 5), Ivanova et al. (6) and Suntsov et al. (7): forests, dry lands near forests, non-flooded lands, paddy fields and irrigated/flooded agricultural lands, houses.
Results
Total host sample size varies widely among rodent species, from 5,683 Rattus tanezumi to 9 Mus musculus. This last species was removed from the analysis due to its low sample size. This great variability can be explained by the variability in abundance of each species but also by unequal sampling among habitats. All rodent species have not been screened for all selected microparasites, with number of pathogens investigated varying according among rodent species (Table 1). The detection of microparasites then depends on both the pathogens’ screening effort (number of pathogens tested) and the host sampling size (number of individual hosts trapped and tested for a given microparasite).
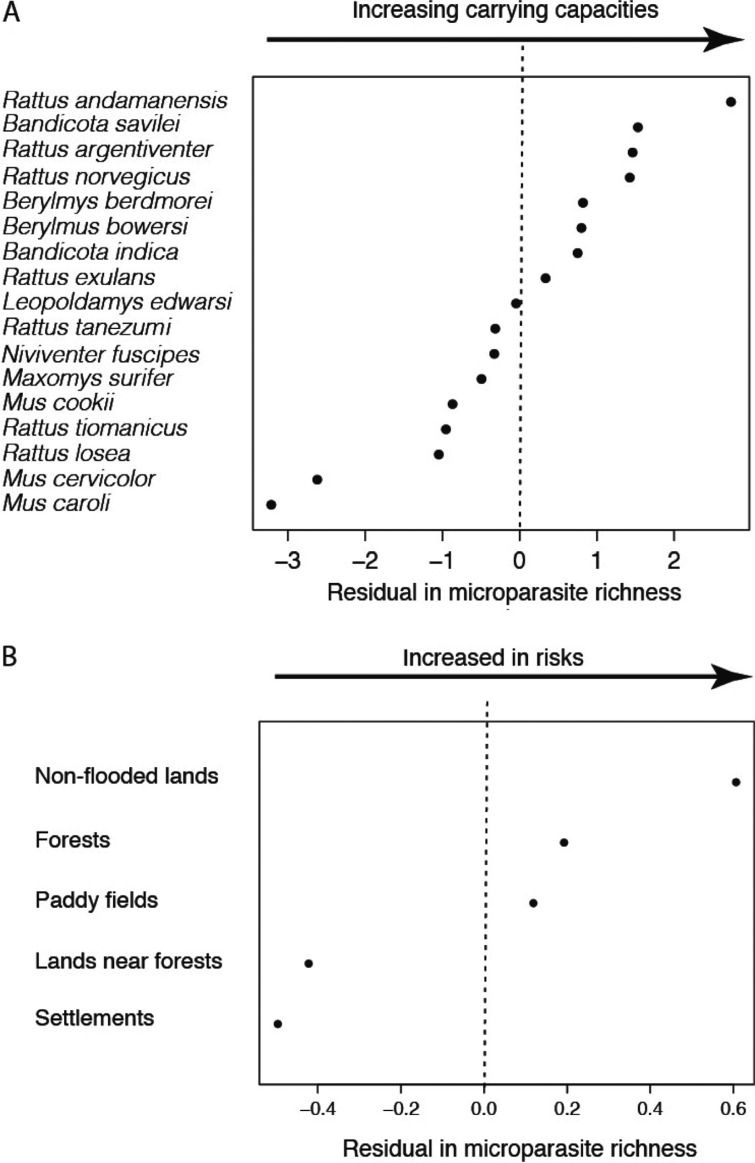
Using multiple regression analysis between microparasite richness and host sample size and pathogens’ screening effort as independent variables, we found that microparasite species richness was positively related to both independent variables (P < 0.0001, host sample size being log transformed). By investigating the residual variations among rodent hosts (Fig. 1B), we show that several rodent species harboured more pathogens than that was expected by the regression model (i.e. positive residual values), particularly Rattus adamanensis, Bandicota savilei, R. argentiventer and R. norvegicus. Two species appeared to harbour less pathogen species than expected by the regression model (i.e. negative residuals values): Mus cervicolor and M. caroli.
Fig. 1.
Residual values in microparasite richness, corrected for rodent sample size and pathogens’ screening effort, positively sorted according (A) to rodent species and (B) to habitats. High positive values of residuals in microparasite richness indicate higher species diversity of microparasites than expected by the regression modelling and can help at identifying ‘good’ rodent carriers of pathogens or risky habitats, at least in term of high diversity of pathogens than can be encountered herein.
Similar reasoning on habitats suggests that higher pathogen richness than expected from correlation with sampling effort (i.e. positive residual values) are found in non-flooded lands, forests and paddy fields.
Conclusions
We have developed here a simple method for prioritizing/targeting rodents that are best carriers of rodent-borne diseases, in a sense that they harbour more pathogen species that expected on the basis of the relationship between pathogen richness and sampling effort. Controlling sampling effort in comparative analysis is usual as pathogen/parasite richness is highly correlated with the efforts done to sample their hosts. However, host sample size is also often positively correlated with some host features such as host density or host geographical range (8). A host living in high density and a wide geographical range is more sampled than a host living in low density and on restricted range. As parasite/pathogen richness is found correlated with host density and/or host geographical range (9, 10), there are potential confounding effects between these host features and the sampling effort to detect these parasites/pathogens. Here, rather to determine the potential determinants of pathogen richness in rodents, which is a difficult task due to the lack of knowledge on many life traits of the species living in Southeast Asia, we used the residual variations of the pathogen richness/sampling effort relationship. Then, high residuals values mean high pathogen richness whatever the explaining factor. Interestingly, our results suggest some rodent species that are not commonly investigated to target for pathogen screening or surveillance such as R. adamanensis or B. savilei.
The second result suggest that non-flooded lands and forests should be more taken into caution, whereas much surveys focused on paddy rice fields and households, although for obvious reasons. There is growing empirical evidence that some ecosystems are prone to alter or improve parasite transmissions (11–13). The recent study of Duffy et al. (14) emphasizes that ecosystems with high productivity (and then high host densities) could select hosts for being more resistant to infections to limit epidemics and parasite transmission but also less fecund (due to trade-offs between reproduction and immunity) compare to host living in ecosystems with low productivity. Assuming the reality of differences in pathogen richness between rodent species and the various habitats, our results call for future studies in Asian ecosystems to improve the processes prone to explain such patterns.
Finally the simple method developed here based on known research effort in pathogens’ screening of wildlife can present some interest in surveillance prioritization (15) by allocating wildlife surveillance effort to specific rodent species in specific habitats.
Conflict of interest and funding
The authors have no conflicts of interest. This study is supported by the French ANR Biodiversity ANR 07 BDIV 012, project CERoPath, ‘Community Ecology of Rodents and their Pathogens in a changing environment’.
References
- 1.Sejvar J, Tangkanakul W, Ratanasang P, Dowell SF, Sangjun N, Bragg S, et al. An outbreak of leptospirosis, Thailand – the importance of the laboratory. Southeast Asian J Trop Med Public Health. 2005;36:289–95. [PubMed] [Google Scholar]
- 2.Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Critic Rev Microb. 2009;35:221–70. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 3.Sodhi NS, Koh LP, Brook BW, Ng PKL. Southeast Asian biodiversity: an impending disorder. TREE. 2004;9:654–60. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Jittapalapong S, Inpankaew T, Sarataphan N, Herbreteau V, Hugot JP, Morand S, et al. Molecular detection of divergent trypanosomes among rodents of Thailand. Infection Gen Evol. 2008;8:445–9. doi: 10.1016/j.meegid.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Jittapalapong S, Sarataphan N, Maruyama S, Hugot JP, Morand S, Herbreteau V. Seroprevalence of Toxoplasma gondii infections of rodents in Thailand. Vector-Borne Zoonot Dis. 2011;11:231–7. doi: 10.1089/vbz.2009.0238. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova S, Herbreteau V, Blasdell K, Chaval Y, Buchy P, Guillard B, et al. Leptospira and rodents in Cambodia: environmental determinants of infection. Am J Trop Med Hyg. 2012 doi: 10.4269/ajtmh.2012.11-0349. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suntsov VV, Ly TVH, Adler GH. Distribution of rodents along a gradient of disturbance on the Tay Nguyen Plateau of southern Vietnam. Mammalia. 2003;67:379–83. [Google Scholar]
- 8.Guégan JF, Kennedy CR. Parasite richness/sampling effort/host range: the fancy three-piece jigsaw puzzle. Parasitol Today. 1996;12:367–70. doi: 10.1016/0169-4758(96)10054-5. [DOI] [PubMed] [Google Scholar]
- 9.Poulin R, Morand S. The diversity of parasites. Quart Rev Biol. 2000;75:277–93. doi: 10.1086/393500. [DOI] [PubMed] [Google Scholar]
- 10.Bordes S, Morand S. The impact of multiple infections on wild animal hosts: a review. Infect Ecol Epidemiol. 2011;1:7346. doi: 10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottontail VM, Wellinghausen N, Kalko EKV. Habitat fragmentation and haemoparasites in the common fruit bat, Artibeus jamaicensis (Phyllostomidae) in a tropical lowland forest in Panamá. Parasitology. 2009;136:1133–45. doi: 10.1017/S0031182009990485. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie TR, Chapman CA. Forest fragmentation, the decline of an endangered primate and changes in hot parasite interactions relative to an unfragmented forest. Am J Primatol. 2008;70:222–30. doi: 10.1002/ajp.20475. [DOI] [PubMed] [Google Scholar]
- 13.Rohr J, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman J, et al. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–39. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- 14.Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeir CA, Hall SR. Ecological context influences epidemic size and parasite driven evolution. Science. 2012;335:1636–38. doi: 10.1126/science.1215429. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre KM, Hawkes I, Waret-Szkuta A, Morand S, Baylis M. The H-Index as a quantitative indicator of the relative impact of human diseases. PLoS ONE. 2011;6:e19558. doi: 10.1371/journal.pone.0019558. [DOI] [PMC free article] [PubMed] [Google Scholar]