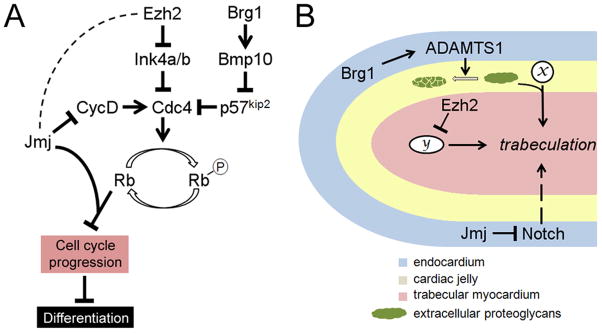
Figure 5. Roles of Ezh2, Brg1 and Jmj in cardiomyocyte proliferation and trabeulation.
(A) Pathways by which Ezh2, Brg1 and Jmj regulate fetal cardiomyocyte proliferation. Cyclins (such as cyclin D) activates cyclin-dependent kinases (such as Cdc4), which phosphorylate Rb and relieve Rb repression of a number of genes essential for cell cycle progression. Ezh2 promotes fetal cardiomyocyte proliferation by direct repression of the cyclin-dependent kinase inhibitor Ink4a/b. Brg1 also promotes fetal cardiomyocyte proliferation, and it does so by activating Bmp10, which in turn represses another cyclin-dependent kinase inhibitor p57kip2. Jmj inhibits fetal cardiomyocyte proliferation by repressing cyclin D and by acting as a co-repressor for Rb. It is unclear whether Jmj and Ezh2 functionally interact with each other in the repression of cyclin D and Ink4a/b, and if they do, whether Jmj promotes or inhibits PRC2 activity in these contexts (hence dashed line between Jmj and Ezh2). Over-proliferation of fetal cardiomyocytes may result in delayed differentiation. (B) Regulation of trabeculation by Ezh2, Brg1 and Jmj. The diagram shows a trabecula. Formation of these finger-like trabeculae is induced by signaling between the endocardium and the myocardium. Proteoglycans in the cardiac jelly modulates the trabeculation process by modulating the function of signaling molecules (x). Ezh2 expression in the myocardium is required for trabeculation, possibly by repressing an as-yet unidentified downstream effector (y). Brg1 expression in the endocardium promotes termination of trabeculation by activating the secreted proteinase ADAMTS1, which mediates the degradation of extracellular proteoglycans. Jmj expression in the endocardium negatively regulates trabeculation by repressing Notch.