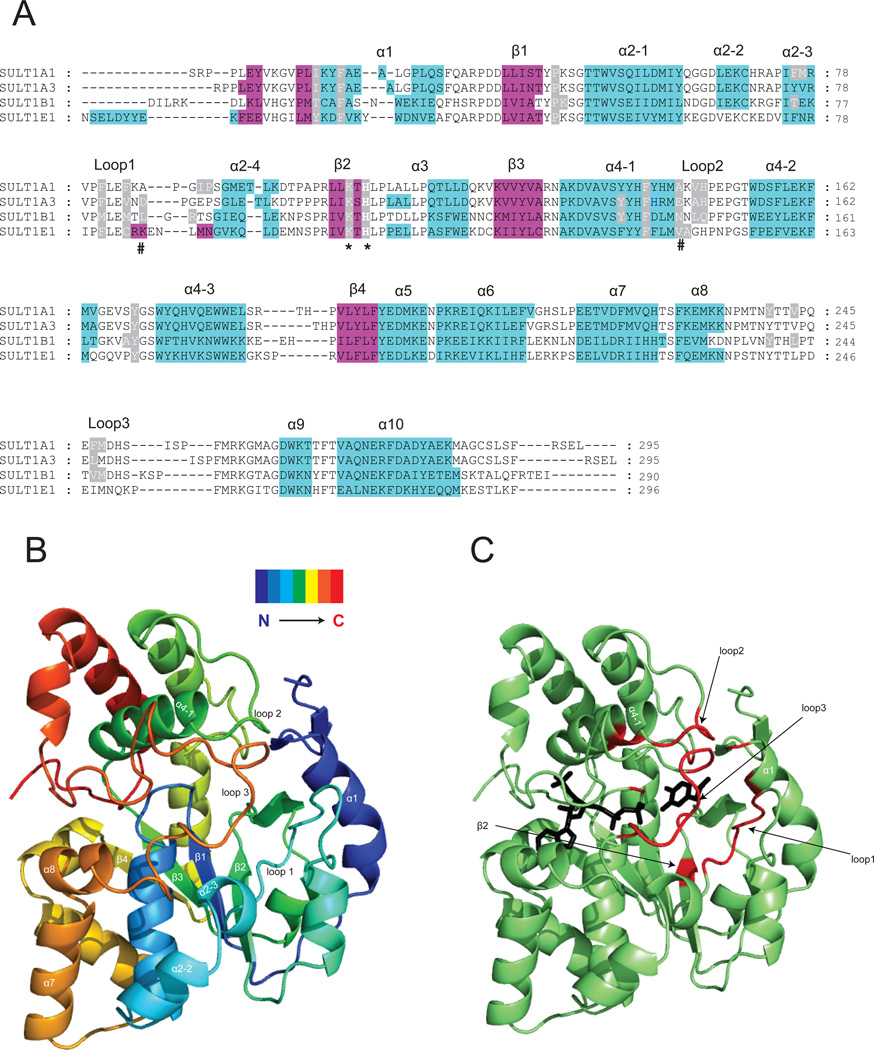
Figure.2. Crystal structures of SULT1 isoforms reveal their highly conserved 3D structure.
Panel A: Structure-based sequence alignment of four crystallized SULT1 isoforms, the α-helices are in cyan and β-strands in magenta. The alignment was performed by overlay of the 3D structures. The conserved catalytic residue histidine and lysine are marked with an asterix. The residues determining the regioselectivity of SULT1A3 towards dopamine and D-dopa [15] are marked with a pound sign. Residues reported to form part of the acceptor pocket are highlighted in grey. Panel B: Ribbon diagram of the SULT1A3 crystal structure [15] showing the 3D folding of elements of secondary structure in a spectrum-colored mode. Panel C: Ribbon diagram of the SULT1A3 crystal structure [15]. The regions that form the substrate-binding pocket are shown in red. The PAPS and substrate (dopamine) are shown as black stick models.