Figure 2.
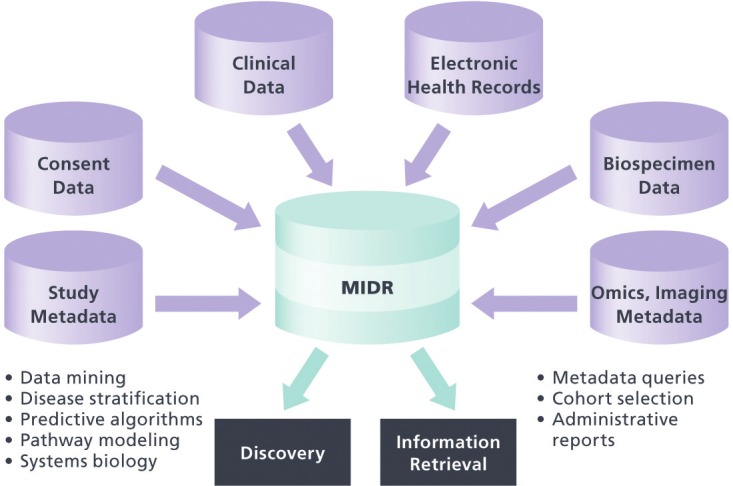
The MURDOCK Integrated Data Repository (MIDR). In addition to the clinical data stored in the current version of the MIDR, the next release will include subject consent data and structured metadata for study protocol, biospecimens, -omic, and imaging data. The resource will enable both administrative information retrieval and scientific discovery through hypothesis generation.
