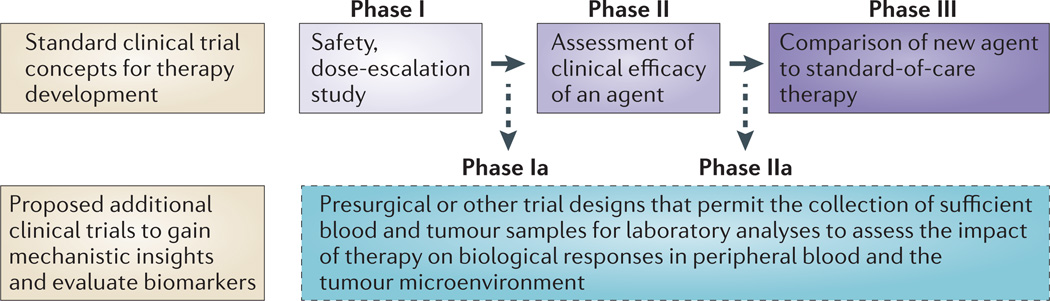
Figure 3. Clinical trial concepts.
The standard paradigm for clinical trial design involves Phase I studies to assess the safety of an agent or therapy, Phase II studies to assess the clinical efficacy of an agent or therapy and Phase III studies to compare the agent or therapy with already established standard-of-care agents or therapies. We propose including Phase Ia and Phase IIa studies as a part of the standard paradigm for cancer immunotherapy trials, thereby allowing the evaluation of biological and immunological responses to an agent or therapy in both peripheral blood and tumour tissues.