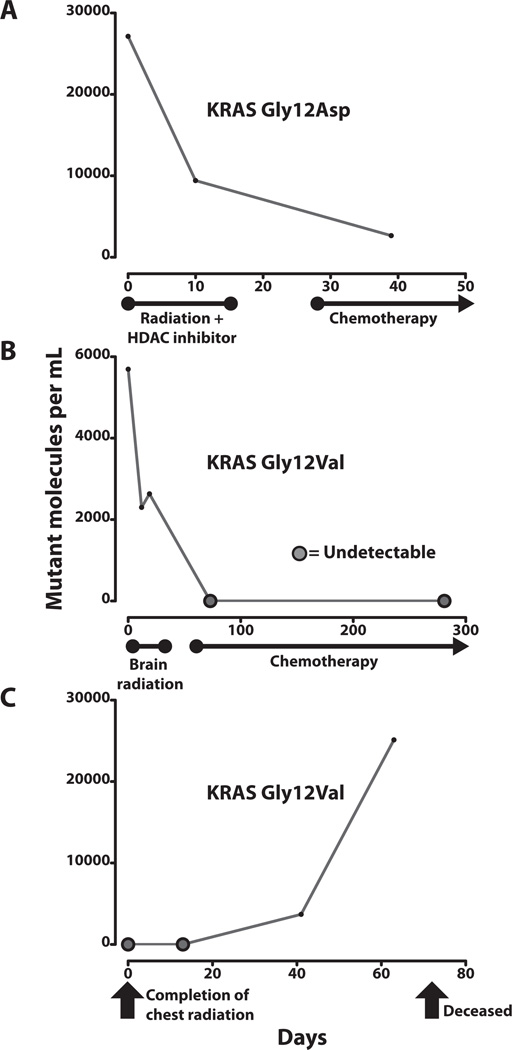
Fig. 4. Changes in ctDNA levels with treatment or disease progression.
Measurements of mutant ctDNA from patients with NSCLC are shown at various times in relation to therapeutic interventions and disease status. ctDNA was considered undetectable if sequence counts yielded a quantity of less than one mutant molecule per sample. Median genome equivalents per sample, as determined by real-time PCR were 9602 (IQR = 5412–11513) (A) Patient 3 had stage IV lung adenocarcinoma with a 4.3 cm right upper lobe tumor and large metastases in the abdomen and supraclavicular region. She was treated concurrently with an experimental histone deacetylase (HDAC) inhibitor and palliative radiation therapy directed at her painful 6.9 cm supraclavicular lesion. She began chemotherapy treatment shortly afterwards. (B) Patient 5 had a 7.5 cm lung adenocarcinoma with eight small brain metastases ranging from 3 mm to 15 mm in size at presentation. He was treated with palliative whole-brain radiation therapy, followed by long-term weekly chemotherapy. Follow-up imaging revealed an excellent, durable response with shrinkage of the lung tumor to ~15% of its original volume at 7 months after diagnosis. No evidence of disease progression was seen during this time period. (C) Patient 9 underwent definitive radiation treatment for locally advanced, stage IIIB undifferentiated NSCLC. Other health conditions prevented him from undergoing surgery or concurrent chemotherapy. Blood sample collection commenced upon completion of his treatment. Although his disease was confined to the thorax prior to initiating radiation therapy, a PET scan performed 8 weeks after treatment showed marked progression of disease with multiple osseous, hepatic, and subcutaneous metastases. He expired 10 weeks after completing treatment.