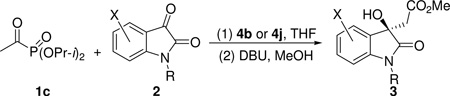
Table 2.
Substrate Scope of the Direct Aldol Reactiona
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | X | R | 3 | time (h) | yield (%)b | ee (%)c | |
| 1 | H | Tr | 3d | 6.0 (6.0) | 80 (83) | 94 (95) | |
| 2 | 4-Cl | Tr | 3e | 5.5 (4.0) | 81 (87) | 8d (96) | |
| 3 | 4-Br | Tr | 3f | 5.0 (4.5) | 70 (92) | 21d (96) | |
| 4 | 5-Me | Tr | 3g | 4.0 (4.5) | 92 (94) | 98 (95) | |
| 5 | 5-MeO | Tr | 3h | 4.0 (4.0) | 90 (88) | 94 (94) | |
| 6 | 5-F | Tr | 3i | 3.0 (4.0) | 88 (93) | 95 (90) | |
| 7 | 5-Cl | Tr | 3j | 4.0 (3.5) | 84 (86) | 95 (90) | |
| 8 | 5-Br | Tr | 3k | 4.0 (3.0) | 89 (92) | 93 (89) | |
| 9 | 5-I | Tr | 3l | 3.0 (2.0) | 91 (93) | 94 (89) | |
| 10 | 5-NO2 | Tr | 3m | 4.5 (4.5) | 88 (85) | 84 (72) | |
| 11 | 6-Br | Tr | 3ne | 4.0 (3.0) | 92 (94) | 97 (94) | |
| 12 | 7-Br | Bn | 3oe | 3.0 (3.0) | 91 (92) | 93 (88) | |
| 13 | 5,7-Br2 | Bn | 3pe | 1.0 (1.5) | 85 (88) | 93 (82) | |
| 14 | 5,7-Me2 | Bn | 3qe | 5.0 (5.0) | 82 (80) | 94 (92) | |
Unless otherwise specified, all aldol reactions were conducted with diisopropyl acetylphosphonate (1c, 0.50 mmol), isatin (2, 0.1 mmol), and catalyst 4b or 4j (0.0050 mmol, 5 mol %) in THF (1.0 mL) at −15 °C under a nitrogen atmosphere. After the aldol reaction was completed, the reaction mixture was treated with DBU (0.10 mmol) and MeOH (1.0 mL) at rt for 15 min to convert the original reaction product to compound 3 in situ. Data in parentheses are those of catalyst 4j.
Yield of isolated compound 3 after column chromatography.
Unless otherwise noted, ee values were determined by HPLC analyses using a ChiralCel OD-H column. The absolute configuration of the major enantiomer of compound 3o was assigned by X-ray crystallographic analysis. The configuration of the rest compounds was assigned on the basis of the reaction mechanism.
The S-enantiomer was obtained as the major product.
Determined by HPLC analysis using a ChiralPak AD-H column.
