Abstract
California serogroup viruses, including Jamestown Canyon virus (JCV) and snowshoe hare virus (SSHV), are mosquito-borne members of the Bunyaviridae family and are endemic across North America. These arboviruses are potential pathogens which occasionally cause neuroinvasive disease in humans and livestock. A neutralization assay was used to document JCV and SSHV seroprevalence using blood collected from a variety of domestic and wildlife host species. These species were sampled in an island setting, Newfoundland, which contains diverse ecoregions, ecological landscapes, and habitats. Seroprevalence rates for each virus differed significantly among host species and within certain species across different geographic areas. JCV was significantly associated with large mammals, and SSHV was significantly associated with snowshoe hares. Seroprevalence rates in the 5 species of animals tested for prior exposure to JCV ranged from 0% in snowshoe hares to 64% in horses. Seroprevalence rates for SSHV ranged from less than 1% in bovines to 55% in all snowshoe hares. The seroprevalence of SSHV differed significantly (P < 0.05) among hares occupying the discrete habitats of watersheds separated by 14 to 35 km. Cattle on farms in boreal forest landscapes displayed significantly higher JCV seroprevalence (P < 0.001) than those on farms located in seacoast landscapes. Lifelong geographic isolation of cattle to insular Newfoundland was associated with significantly lower JCV seroprevalence (P < 0.01) than that for cattle which had lived off-island.
INTRODUCTION
Snowshoe hare virus (SSHV) and Jamestown Canyon virus (JCV) are mosquito-borne pathogens belonging to the California serogroup of bunyaviruses (genus Orthobunyavirus, family Bunyaviridae). Characteristic of bunyaviruses, these viruses are enveloped and composed of three single-stranded negative-sense RNA segments. SSHV and JCV multiply in both vertebrates and arthropods and are transmitted in saliva to a vertebrate host when an infected arthropod takes a blood meal (3, 5). SSHV and JCV are also potential pathogens that are capable of causing neuroinvasive disease and other illnesses in both humans and livestock (4, 17). Although related as members of the California serogroup, SSHV and JCV are antigenically and phylogenetically distinct California serogroup viruses (CSV) exhibiting nucleotide and amino acid sequence divergence of 20 to 30% when small (S) and medium (M) RNA segments are compared (11).
Serology is a tool routinely used to determine the exposure of an animal to a virus. It identifies the presence of antibodies to a specific virus produced by the host organism. Previous serological studies have determined the range of California serogroup agents in Canada (5), including Manitoba (26), Ontario (7), New Brunswick, Nova Scotia, and Prince Edward Island (15, 27, 28). In Newfoundland and Labrador, an initial serosurvey (29) confirmed the circulation of SSHV and JCV in Newfoundland, with seroprevalence rates of 8.6%, 20.9%, and 54.5% in humans, horses, and hares, respectively. Past mosquito surveys conducted in Newfoundland have detected the presence of several species that transmit CSV (21, 29, 32, 33, 39). Common among these confirmed vectors of JCV and SSHV are Ochlerotatus canadensis, Ochlerotatus communis, Ochlerotatus excrucians, and Ochlerotatus punctor (2, 5). Other known CSV vectors recorded on the island include Culiseta morsitans, Culiseta melanura, Aedes cinereus, and many other mosquito species of low frequency of occurrence.
While many wild and domestic mammal species have been shown to be seropositive for CSV (5, 17) and can be referred to as “sentinels” for virus circulation, the majority of these probably do not develop significant viremias and therefore contribute insignificantly to virus amplification. However, snowshoe hares, squirrels, and small mammals are generally considered the primary amplification hosts of SSHV (5). White-tailed deer are the primary reservoir and amplifying hosts of JCV where they occur, and wild free-ranging ungulates such as moose and bison are believed to be the primary reservoir and amplifying hosts of JCV in other locations (17, 18, 19, 22). Studies evaluating host feeding patterns in various mosquito species which transmit JCV and SSHV also indicate that these vectors acquire blood meals from deer and small mammals such as squirrels and chipmunks (30). Molecular DNA analyses of blood meals have also identified mule deer and elk as hosts for snow-melt mosquitoes that carry JCV (31).
The transmission and spread of viral agents are influenced by both the demographics of host populations and the geographic structures of the environments they occupy (23). Viruses are present in all environments, but the significance of their ecological roles can best be understood in marine ecosystems such as those in which virus-induced mortality of microorganisms promotes the turnover of dissolved organic matter and influences global biogeochemical cycles (34, 37). Less is known of the ecological roles of viruses in most ecosystems. Temporal changes in climate and arthropod habitat can influence the movement of viral agents (10). Viruses, like other disease agents of animals, have enormous capability for rapid evolution and dissemination (12).
Islands have previously provided instructive settings to understand the dissemination and abundance of organisms (25, 36). Newfoundland is a large island (108,860 km2) located in the northwest Atlantic Ocean. It has 9 distinct ecological regions (14). This environmental heterogeneity makes it an ideal setting to conduct surveillance on the transmission and spread of viral agents and the factors that might influence these during a period of environmental change.
The purpose of this study was to better understand the ecological basis of CSV seroprevalence on this large island landscape. First, we needed to measure the prevalence and significance of these viruses in a number of potential host species. Blood samples were collected from a range of agricultural and wildlife species in Newfoundland and Labrador. Samples seropositive for SSHV and JCV antibodies were analyzed to assess the role of different domestic animals and wildlife as hosts or sentinels of these viruses. The collection of data across several species was also the best approach to determine if exposure to these agents might result in clinical cases of California serogroup encephalitis or other animal disease.
Investigation of the latter involved examination of the geographic variation in the seroprevalence of these viruses. Comparison of viral activities among different ecological regions within the island provided the opportunity to examine how concepts of landscape ecology relate to viruses. Data from 3 host species were used separately to examine variation at broad spatial scales in seroprevalence of these viruses. Blood sera were collected in 2008 from horses on the Avalon Peninsula in eastern Newfoundland and the Humber River Basin in western Newfoundland, 2 large and different ecological landscapes separated by more than 300 km. Variation at a smaller scale within one ecoregion was examined using blood sera collected from wild snowshoe hare populations at three habitats in western Newfoundland. These samples were collected from animals in different watersheds separated by 15 to 35 km. The third data set of serum antibodies was available from a subset of bovines (dairy cattle) born and raised on the island and distributed on seven farms in western Newfoundland. These farm settings were selected to permit comparisons of seroprevalence in animals located at farms in two ecologically different landscapes, an inland boreal freshwater catchment and a marine coastline.
Finally, the island setting of this research also facilitated a test of the effect of isolation on the seroprevalence of CSV activity. Levels of seroprevalence of JCV and SSHV in animals living geographically isolated on the island of Newfoundland were compared with those in animals which have traveled off-island to the mainland of North America. This was tested using separate data sets for dairy cattle and horses from western Newfoundland. This analysis was designed to determine if the 95-km-wide marine Gulf of St. Lawrence provides any degree of isolation from these CSV pathogens.
MATERIALS AND METHODS
Study design.
The primary blood sampling was done during the fall of 2008 at sites, i.e., farms, on the west coast of the island of Newfoundland. These cattle and mink farms were participants in a routine agricultural herd health monitoring protocol. Farms where data were collected were selected so as to represent contrasting ecological environments. Wildlife blood serum samples were simultaneously collected in a study designed to sample snowshoe hares distributed in three discrete watershed habitats. In order to increase the range of potential CSV host species considered, the original design was supplemented by a selection of random samples of frozen sheep blood sera collected across the island in 1997 and held in an archival serum bank (−70°C) at the Animal Health Division of the Newfoundland and Labrador (NL) Department of Natural Resources. Horse serum samples collected within the Humber River Basin in western Newfoundland for host species consideration were compared with samples collected in the Avalon Peninsula region located on the eastern end of the island. Figure 1 indicates the locations of the species sampled in 2008. Protocols for the blood sampling and restraint of domestic animals and the capture and release of wildlife were submitted for animal care review and approval at Memorial University.
Fig 1.
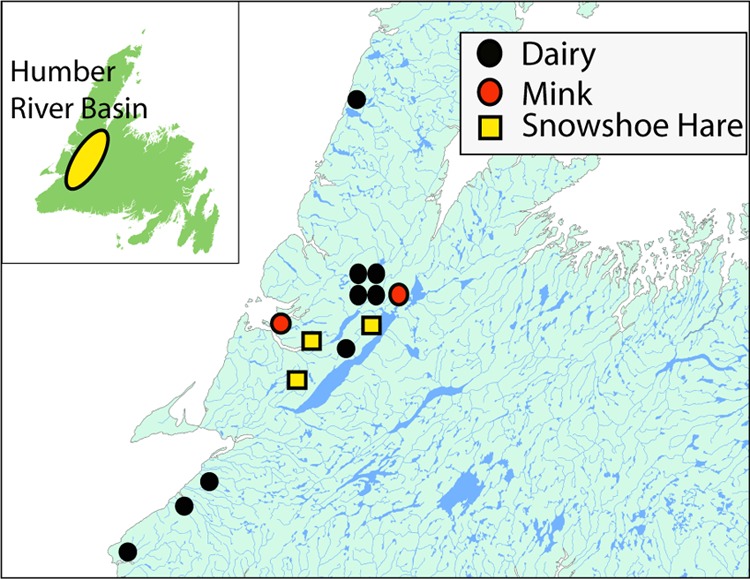
Blood sample locations near the Humber River Basin in western Newfoundland in 2008. Samples from horses were collected within this region and on the Avalon Peninsula, over 300 km eastward. Random samples of frozen sheep blood sera collected across the island in 1997 were obtained from an archival serum bank (−70°C) at the Animal Health Division of the Newfoundland and Labrador (NL) Department of Natural Resources in St. John's.
Blood samples (10 ml) from horses were drawn by regional veterinarians. Twenty-four originated on the Avalon Peninsula in eastern Newfoundland and 27 were collected within the Humber River Basin on the west coast of the island. These locations represent distinct ecological regions over 300 km apart. These samples were taken to a laboratory facility where serum and cell fractions were separated within 24 h using a clinical centrifuge. The serum fraction was transferred to replicate labeled test tubes and frozen at −29°C. Within 4 weeks, these frozen samples were shipped to the NL Animal Health Division serum bank for permanent archive. Within 1 month, one serum sample from each animal tested was sent to the Viral Zoonoses section at the National Microbiological Laboratory (NML) of the Public Health Agency of Canada in Winnipeg for serological analysis.
Horses lived under a variety of conditions, from solitary to being housed in riding stables or as a herd on summer pasture. Each animal owner provided a signed consent form which identified details of this study and assigned authority for use of sera collected.
Thirty dairy cattle were sampled from each of 9 farms along the west coast of Newfoundland by regional veterinarians during the course of routine dairy herd health monitoring in 2008. Samples were processed as previously described. Three replicate samples were forwarded to the provincial serum bank for archive, one of which was couriered to the NML for analysis. Samples from 253 cattle collected in 2008 and 66 collected during 2000 to 2003 and maintained in the serum bank were analyzed for total CSV seroprevalence.
Two hundred two blood samples (1.0 ml) were collected from 2 commercial mink farms in western Newfoundland by researchers at Grenfell Campus of Memorial University with the assistance of herd managers. Blood samples were centrifuged, and sera were frozen in 0.5-ml aliquots and transported as previously described.
Twenty individual wild snowshoe hare blood samples were available to include in the analyses. These were obtained using live traps at 3 field sites, located in 3 different watersheds in western Newfoundland. Corner Brook Lake, Pynn's Brook, and Hughes Brook are characterized by black spruce-kalmia forest, mixed hardwood forest, and balsam fir and black spruce-balsam fir forest. These habitats are separated by distances ranging from 14 to 35 km. The live trapping caught 5, 9, and 6 hares in each watershed, respectively. This trapping involved visiting baited traps on a daily schedule, drawing 1.0-ml blood samples, and releasing the wild caught hares unharmed. Confirmation of the success of this method was provided by inspection of healthy retrapped marked hares over 2 weeks later. Blood sera were processed as described above.
In order to increase the number of sentinel species considered by this study, 119 frozen sheep sera collected in 1997 and stored in the blood serum bank (−70°C) were also submitted for analyses.
As farm biosecurity around the risk of disease transmission among farms was a concern, biosecurity protocols ranging from boot dips and hand sanitation to the use of disposable overalls and bootees were employed as required during sampling.
Analyses: identification of samples seropositive for viral antibodies.
The NML screened the sera for the presence of SSHV and JCV neutralizing antibodies. Testing was carried out using a plaque reduction neutralization test (PRNT) at 1:20 dilutions of the collected sera for initial screening for CSV antibody (8). In brief, mixtures of virus (either SSHV or JCV) and dilutions of animal serum were incubated at 37°C for 1 h in tissue culture media and then added to 6-well plates containing Vero cell monolayers. After the plates had been incubated at 37°C for 1 h, a nutrient agar overlay was added. The plates were placed in a CO2 incubator for approximately 3 days, after which another overlay, containing neutral red as a vital stain, was added. The plates were then checked periodically over the next 1 to 2 days for plaque formation. Serum samples inhibiting at least 90% of possible plaque formation relative to virus controls were deemed positive for CSV antibodies. Further endpoint titrations following serial dilutions were carried out to differentiate between exposures to SSHV or JCV using a ≥4-fold determination to identify specific virus antibodies and exposures. The highest serum dilution with a plaque reduction of at least 90% was defined as the titration endpoint.
Statistical analyses.
Contingency table analyses (41) were used initially to compare the prevalences of CSV antibodies in samples of blood sera collected from cattle, horses, mink, sheep, and snowshoe hares. The proportion of blood sera positive for antibodies was used as the measure of prevalence. The initial null hypothesis considers the prevalence of exposure to CSV equal in all species tested. A chi-square test of independence was applied to the actual frequencies of positive data and sample sizes for each species.
A significant chi-square result at the level of a P value of <0.05 was followed by a post hoc Tukey-type multiple-comparison test for proportions of the serogroup prevalence values across species (41). This involved ranking the prevalence values for each species. An angular transformation was applied to each proportion, and the calculated q values of the pairwise comparisons were tested against a critical value of the q (studentized range) distribution.
Subsequent analyses further examined the prevalence of the specific virus antibodies (JCV and SSHV) in the host species. Contingency table analysis of seropositivity data used a chi-square test followed by a post hoc Tukey-type multiple-comparison test where appropriate (41).
These data were used to examine the nature of geographic variation in the seroprevalence of these viruses and to provide an indication of the extent of virus circulation in a particular region or site. To analyze viral activity between different ecological regions and landscapes within the island, or among different habitats, a G test using the log likelihood ratio was used. The G statistic calculated in this test approximates the distribution of the chi-square and was tested against a table of critical values of the chi-square distribution. This analytical approach was used to test differences in the seroprevalence of JCV in host cattle which had been raised “isolated” on the island of Newfoundland compared with those that had been reared on or traveled to the mainland of North America.
To test seropositivity differences of host cattle in boreal freshwater versus seacoast landscapes, only the subset of cattle at these farms which had never been off the island of Newfoundland were selected. This eliminated any potential influence of viral exposure acquired off-island.
RESULTS
Serological procedures such as PRNTs can be used to determine exposure of animals to specific CSV. The proportion of blood sera positive for antibodies was used as the measure of prevalence (6). Neutralization assays such as PRNTs primarily detect IgG antibody, which can be present in previously infected animals for several years. However, the actual time frame of exposure based on the presence of neutralizing antibody can be determined only where seroconversion is documented in paired samples.
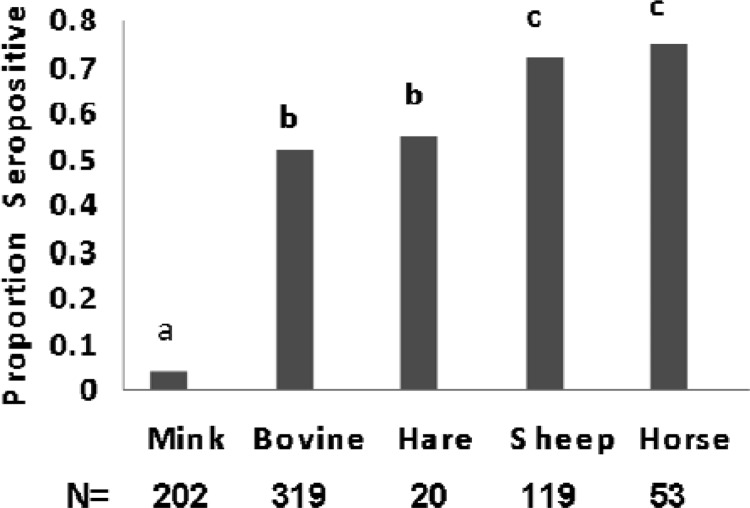
Seroprevalence in the 5 species tested for prior exposure to CSV (either JCV or SSHV or both) ranged from 0.040 in mink to 0.755 in horses (Fig. 2). The contingency table analysis based on actual counts of positive and negative samples for each species identified a significant difference (χ2 = 192, P < 0.001) in the seroprevalence of CSV among the species tested. The subsequent Tukey-type multiple comparisons of the ranked seropositives revealed that the proportion of mink seropositives was significantly lower (P < 0.001) than that of all other species. The proportion of bovines seropositive (0.533) for total CSV antibodies was not significantly different (P > 0.05) from that of snowshoe hares (0.550). Levels of seropositivity for total CSV antibodies in sheep (0.723) did not differ significantly from those in horses (0.755). However, CSV seropositivity did differ significantly between both of these pairs of species (P < 0.001).
Fig 2.
Seroprevalence for prior exposure to CSV (both JCV and SSHV) indicates the proportion of blood sera positive for antibodies to CSV. Different letters indicate significant differences (P < 0.001) in proportion seropositive using a Tukey-type multiple comparison for proportions (41).
To examine the association of the seroprevalences of specific CSV, JCV and SSHV, the differences in the proportion of each species containing antibodies to these specific viruses were analyzed. This test was applied to data collected in 2008 following serial dilutions and endpoint titrations to differentiate between exposures to JCV and SSHV.
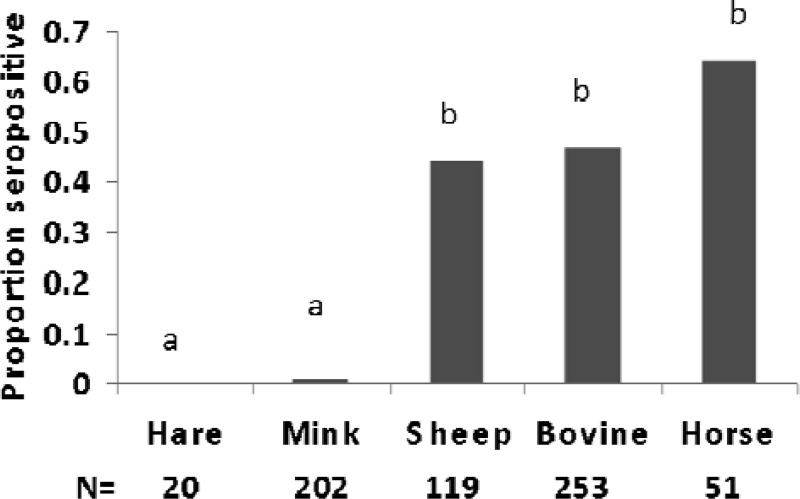
Seroprevalence to JCV ranged from 0.00 in hares to 0.643 in horses (Fig. 3). The contingency table analyses identified a significant difference in seropositivity among 4 species sampled in 2008 (chi-square = 120, P < 0.001). The post hoc Tukey-type multiple comparison indicated no significant differences between the JCV values of snowshoe hares (0.00) and mink (0.10) (P > 0.05). JCV seropositivity did differ between bovines (0.470) and horses (0.643) (P < 0.01). However, seropositivity for JCV in Fig. 3 displays the highly significant difference between both of these pairs of species (P < 0.001).
Fig 3.
Seroprevalence for prior exposure to JCV indicates the proportion of blood sera positive for antibodies to JCV. Different letters indicate significant differences (P < 0.001).
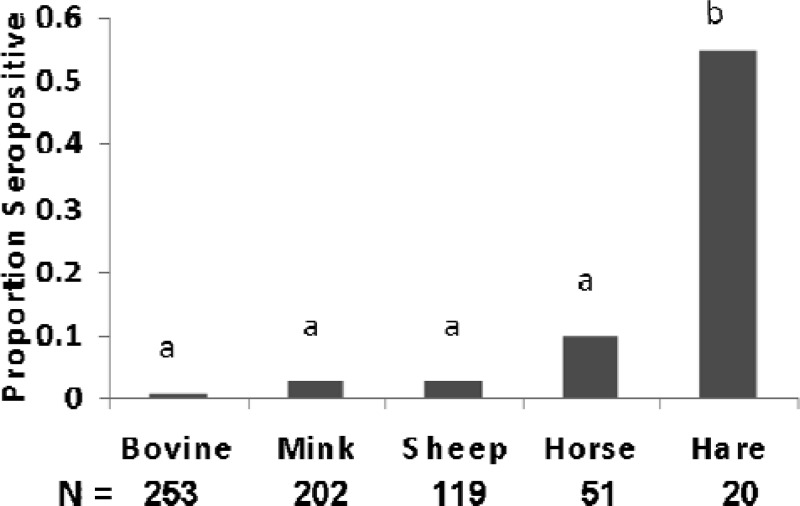
SSHV seroprevalence displayed a different pattern of infectivity in the 4 species sampled in 2008. Values ranged from 0.008 in bovines to 0.55 in snowshoe hares (Fig. 4). Contingency table analyses indicated that SSHV seropositivity was significantly different across these species (chi-square = 120, P < 0.001). The post hoc multiple comparison indicated that SSHV seropositivity in snowshoe hares (0.55) was significantly higher (P < 0.001) than that in all other species. SSHV seropositivity was significantly lower (P < 0.001) in cows (0.008) than in horses (0.102) and hares but not significantly different from the antibody prevalence in mink (0.03). SSHV seropositivity values for mink and horses were not significantly different.
Fig 4.
Seroprevalence for prior exposure to SSHV indicates the proportion of blood sera positive for antibodies to SSHV. Different letters indicate significant differences (P < 0.001).
These initial analyses indicate that JCV antibodies are primarily associated with bovines, horses, and sheep while SSHV antibodies are primarily found in snowshoe hares. Subsets of appropriate data in these species were selected to examine geographic variation in the seroprevalences of these viruses (Table 1). With the results of these binomial comparative trials, the log likelihood ratio (G statistic) was used to test among areas for differences in the proportions of samples with and without antibodies to JCV or SSHV.
Table 1.
Effects of various scales of geographic separation on seroprevalence of CSV tested in three different host species in Newfoundland
| Scale of geographic separation (km) | Host species | Virus | Locationa | Virus seroprevalence by location | G statistic | P value |
|---|---|---|---|---|---|---|
| Region (>300) | Horse | JCV | Avalon peninsula | 0.557 | 0.792 | >0.05 |
| Humber River basin | 0.661 | |||||
| Watershed (14–35) | Hare | SSHV | CBL | 0.0 | 10.252 | <0.05 |
| PB | 0.778 | |||||
| HB | 0.667 | |||||
| Landscape (50–150) | Cattleb | JCV | Boreal forest | 0.561 | 26.216 | <0.001 |
| Marine seacoast | 0.113 |
CBL, Corner Brook Lake; PB, Pynn's Brook; HB, Hughes Brook.
Cattle with lifelong geographic isolation on Newfoundland.
Regional differences between two large and ecologically distinct landscapes were examined by comparing JCV seropositivity in horses in regions over 300 km apart. Analyses indicate no significant difference (G = 0.155, P > 0.05) in the prevalences of exposure to JCV of horses in these east (Avalon Peninsula = 0.557) and west (Humber River Basin = 0.611) regions of Newfoundland.
Snowshoe hares sampled in three distinct watersheds within the Humber River catchment provided data to examine geographic variation in prevalence of viral antibodies on a more restricted geographic scale. The SSHV seropositivity values for snowshoe hares live-trapped in these watersheds differed significantly (G = 10.353, P < 0.01). Twenty snowshoe hare samples were available among three habitats for this analysis. This smaller size is a reflection of the increased degree of difficulty associated with sampling live wildlife compared to commercial domestic animals.
The third examination of geographic variation in seroprevalence compared values in bovines on farms in two distinct ecological landscapes. Four farms within the boreal freshwater Humber River Basin and three farms outside this catchment along a seacoast are separated by 50 to 150 km. Blood sera were analyzed for JCV seropositivity in 110 cattle which had lived exclusively on the island of Newfoundland. This analysis identified a highly significant difference (G = 26.216, P < 0.001) of the seroprevalences to JCV in bovines inhabiting the boreal freshwater landscape (0.561) versus bovines living along the seacoast (0.113) (Table 1).
Data to explore the role of lifelong isolation of animals to an island in seropositivity for JCV was examined in bovine serum samples from dairy farms (Table 2). Cattle which had never been off-island (n = 110) displayed a seroprevalence of JCV of 0.345 versus 0.531 for those which had been born or raised off the island (n = 143). This indicated a significantly higher (G = 8.789, P < 0.01) exposure to JCV for off-island-born or -reared cattle.
Table 2.
Seroprevalence of Jamestown Canyon virus in host animals geographically isolated on the island of Newfoundland compared with those which had traveled off-islanda
| Host | Seroprevalence by movements |
G statistic | P value | |
|---|---|---|---|---|
| Isolated | Off-island | |||
| Cattle | 0.345 | 0.531 | 8.789 | <0.01 |
| Horse | 0.786 | 0.833 | 0.095 | >0.05 |
For cattle, 110 isolated cattle and 143 which had traveled off-island were tested; for horses, 13 of each type were tested.
However, the seroprevalence in Humber River Basin horses which traveled off-island (0.833, n = 13), usually to shows or competitions, was not significantly higher than that in horses (0.786, n = 13) which had never been off the island (G = 0.095, P > 0.05).
DISCUSSION
The results show significant rates of CSV seroprevalence among a number of species of animals examined on the island of Newfoundland. The findings are consistent with previous studies indicating CSV circulation in this eastern province (29). Snowshoe hares, horses, sheep, and bovines exhibited high rates of CSV seropositivity. CSV antibodies were also detected in a small number of sera collected from mink. The detected levels of seroprevalence for both JCV and SSHV indicate ongoing circulation of these potential pathogens on the island of Newfoundland and therefore represent a risk for disease in horses, humans, and other animals (17, 20, 24, 35). During the mosquito season in Newfoundland, CSV should be considered part of the differential diagnosis for presentations of clinical illness in both humans and livestock.
Our subsequent examination of the specific CSV involved, JCV or SSHV, also provided a greater level of insight regarding the roles of these viruses among the different host species. SSHV antibodies were detected primarily in hares. This is consistent with past studies indicating snowshoe hares as the principal vertebrate or amplifying hosts of SSHV, while other small rodents, moose, and horses may serve as secondary vertebrate hosts or sentinels (5, 17). JCV antibodies were more significantly associated with larger domestic animals such as sheep, cattle, and horses than with the smaller mink and hares. Similar results have been observed in other geographic regions where JCV circulates (16).
The differences in CSV seroprevalence among species could relate to a variety of factors. These include species-specific differences in susceptibility and immunological response to infection or variation in the duration of neutralizing antibody generated against the specific virus to which the animal is exposed. Another variable contributing to differences in seroprevalence is the mean age of the species being sampled, as longer-lived animals are at risk for exposure for a longer period. The possibility that individuals may have been exposed to both JCV and SSHV at particular times may result in decreased viremia for one agent and an associated impact on observed antibody titers. Dual-infection experiments with other bunyaviruses have provided results indicating that “superinfection” effects should be a consideration when interpreting serological results (9).
Where these differences in seroprevalence to both CSV (JCV and SSHV) were significant (P < 0.05), host feeding preferences of local mosquito vectors may also have contributed to the differences observed. Mosquito feeding preferences can vary between small mammals such as snowshoe hares and larger livestock such as sheep, horses, and cattle. Although many species of Culiseta, Ochlerotatus, and Aedes mosquitoes carry both SSHV and JCV (2, 5), it is possible that subsets of mosquito species that predominately transmit JCV have preferential feeding behaviors that involve larger animals such as deer, moose, and cattle (30, 31). Vectors transmitting SSHV may acquire blood meals more often from smaller mammals such as squirrels and hares (17). Both factors potentially contribute to the observations and results generated in this study. However, the extensive mosquito collections and virus isolations relating to site-specific vector potential were not a part of this initial experiment.
Although large livestock species such as cattle and horses may not develop sufficient levels of viremia to allow for the infection of mosquitoes upon acquiring a blood meal, limited replication of JCV does lead to a detectable immune response. Consequently, they may not be primary amplifying hosts but instead do serve as good sentinel species for the occurrence of JCV (17). It is likely that large livestock bitten by mosquitoes carrying SSHV may not exhibit significant viremia or are less likely to generate adaptive immune responses, and hence SSHV seropositivity rates remain quite low. Previous studies showing various degrees of viremia and antibody response related to the specific CSV and the type of infected animal are consistent with these conclusions (1, 16, 38). We believe that future studies will determine the abundant moose or caribou as the amplification hosts, or primary vertebrate species, of JCV on Newfoundland.
Mink displayed significantly low seroprevalences of both JCV and SSHV. This indicates a low probability of their susceptibility to these viruses. Despite the high number of individual mink in commercial barns and the accessibility of these well-ventilated facilities to mosquitoes, antibodies to JCV and SSHV were recorded in only 2.7 to 3.5% of the mink sampled. The absence of mink data from the 1980s testing in Newfoundland precludes understanding whether these levels are historically consistent or are responses to changing environmental conditions.
Geographic separation and isolation associated with living on a large island are important elements which may contribute to different CSV seroprevalence levels seen in various animals at different locations. Examining seroprevalences in contrasting Newfoundland landscapes (13, 14, 40) in 3 host species revealed insights on this influence of geographic variation. At the regional scale, the Humber River Basin (HRB) is a large catchment of boreal forest centered on a large freshwater river system. The Avalon Peninsula, over 300 km away, is dominated by a maritime barrens landscape. While seroprevalences of both JCV and SSHV in horses were higher in the boreal catchment, the differences in seroprevalence between these ecoregions were not significant (P > 0.05). This result is not surprising given that horses in Newfoundland are primarily recreational animals which are relocated over significant distances in the course of competitions and sales.
Snowshoe hares were sampled in 3 distinct habitats spatially separated by only 14 to 35 km. These habitats differed in tree species composition and physical characteristic. The black spruce-kalmia species habitat of the Corner Brook Lake watershed represents an open-canopy softwood forest habitat. The live trapping of hares at Pynn's Brook was done in a closed-canopy stand of mixed hardwood comprised primarily of speckled alder and white birch situated near agriculture fields. At Hughes Brook, the black spruce-balsam fir creates a closed-canopy softwood forest. All habitats contained ample sources of water but were associated with distinct nonconnected river drainages. Despite the smaller sample sizes involved, significant differences in seroprevalence (P < 0.05) of antibodies to SSHV were obtained at this spatial scale.
The most significant comparison in geographically based variation of CSV was realized at the landscape scale among that subset of cattle experiencing lifelong residence on the island of Newfoundland. The significantly lower (P < 0.05) seroprevalence of JCV among cattle on farms situated on a wind-exposed seacoast compared with that for cattle on farms located in a wind-protected boreal freshwater landscape clearly indicates the potential of environmental factors to influence the incidence of JCV.
The role of isolation in the relationship between pathogen and host, or viral agent and host, is one that fits well in an ecological context. Observation of the role of lifelong isolation of animals to an island in the seropositivity for JCV was possible because many dairy animals on Newfoundland are raised as calves off-island and are imported to the island as pregnant cows. Normal routes of ecological connectivity are minimal to absent across 150 km of ocean. Island-isolated bovines are generally further geographically restricted to one commercial facility for their entire lives. The most likely reason for the significantly higher exposure to JCV for off-island-born or -reared cattle is associated with increased mosquito diversity and density encountered off-island. This is consistent with the hypothesis (23) that the probability of infectious and susceptible hosts coming into contact should be greater in subpopulations having more dispersal.
Ecology has a profound influence on the level of exposure of host animals to California serogroup viruses. Geographic variation at the scale of large landscape structure and small habitat differences and the role of isolation on a large island both demonstrate a role in determining host seroprevalence of JCV and SSHV in Newfoundland.
ACKNOWLEDGMENTS
We thank the following for valuable assistance: Bev Dawe and Mike Tipple, who collected bovine and horse sera; Adam Legge and Jothan Bessey, who sampled hares and mink; Randy Skinner, who prepared the figures; Cathy Keane, who archived the serum samples; and Kristina Dimitrova, who provided expert technical assistance for the serological procedures.
The research was supported by Humber River Basin of Western Newfoundland and Labrador (project no. 2008-005), the Animal Health Division of the Newfoundland and Labrador Department of Natural Resources, and the National Microbiological Laboratory of the Public Health Agency of Canada.
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Amundson TE, Yuill TM, Defoliart GR. 1985. Experimental La Crosse virus infection of red fox (Vulpes fulva), raccoon (Procyon lotor), opossum (Didelphis virginiana) and woodchuck (Marmota monax). Am. J. Trop. Med. Hyg. 34:586–595 [DOI] [PubMed] [Google Scholar]
- 2. Andreadis TG, Anderson JF, Armstrong PM, Main AJ. 2008. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: a ten-year analysis, 1997–2006. Vector Borne Zoonotic Dis. 8:175–188 [DOI] [PubMed] [Google Scholar]
- 3. Artsob H. 1990. Arbovirus activity in Canada. Arch. Virol. 1990(Suppl 1):249–258 [Google Scholar]
- 4. Artsob H. 2000. Arthropod-borne disease in Canada: a clinician's perspective from the ‘cold zone.' Paediatr. Child Health 5:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artsob H. 1983. Distribution of California serogroup viruses and virus infections in Canada, p 277–290 In Calisher CH, Thompson WH. (ed), California serogroup viruses. Alan R. Liss Inc., New York, NY: [PubMed] [Google Scholar]
- 6. Artsob H, Spence LP, Th'ng C. 1984. Enzyme-linked immunosorbent assay typing of California serogroup viruses isolated in Canada. J. Clin. Microbiol. 20:276–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Artsob H, et al. 1986. Arbovirus infections in several Ontario mammals, 1975-1980. Can. J. Vet. Res. 50:42–46 [PMC free article] [PubMed] [Google Scholar]
- 8. Beaty BJ, Calisher CH, Shope RS. 1989. Arboviruses, p 797–856 In Schmidt NJ, Emmons RW. (ed), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th ed American Public Health Association, Washington, DC [Google Scholar]
- 9. Blackmore CGM, Grimstad PR. 1998. Cache Valley and Potosi viruses (Bunyaviridae) in white-tailed deer (Odocoileus virginianus): experimental infections and antibody prevalence in natural populations. Am. J. Trop. Med. Hyg. 59:704–709 [DOI] [PubMed] [Google Scholar]
- 10. Brownstein JS, Holford TR, Fish D. 2005. Effect of climate change on Lyme disease risk in North America. Ecohealth 2:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell WP, Huang C. 1999. Sequence comparisons of medium RNA segments among 15 California serogroup viruses. Virus Res. 61:137–144 [DOI] [PubMed] [Google Scholar]
- 12. Cox GW. 2004. Alien species and evolution. Island Press, London, United Kingdom [Google Scholar]
- 13. Damman AWH. 1976. Plant distribution in Newfoundland especially in relation to summer temperatures measured with the sucrose inversion method. Can. J. Bot. 54:1561–1585 [Google Scholar]
- 14. Damman AWH. 1983. An ecological subdivision of the island of Newfoundland, p 163–206 In South GR. (ed), Biogeography and ecology of the island of Newfoundland. Junk Publishers, The Hague, Netherlands [Google Scholar]
- 15. Embil JA, Embree JE, Artsob H, Spence L, Rosee KR. 1978. Antibodies to snowshoe hare virus of the California group in the snowshoe hare (Lepus americanus) populations of Nova Scotia. Am. J. Trop. Med. Hyg. 27:843–845 [DOI] [PubMed] [Google Scholar]
- 16. Fulhorst CF, Hardy JL, Eldridge BF, Chiles RE, Reeves WC. 1996. Ecology of Jamestown Canyon virus (Bunyaviridae: California serogroup) in coastal California. Am. J. Trop. Med. Hyg. 55:185–189 [DOI] [PubMed] [Google Scholar]
- 17. Grimstad PR. 1988. California group virus disease, p 99–136 In Monath TP. (ed), The arboviruses: epidemiology and ecology, vol 2 CRC Press, Boca Raton, FL [Google Scholar]
- 18. Grimstad PR, Schmitt SM, Williams DG. 1986. Prevalence of neutralizing antibody to Jamestown Canyon virus (California group) in populations of elk and moose in northern Michigan and Ontario, Canada. J. Wildl. Dis. 22:453–458 [DOI] [PubMed] [Google Scholar]
- 19. Grimstad PR, Williams DG, Schmitt SM. 1987. Infection of white-tailed deer (Odocoileus virgonianus) in Michigan with Jamestown Canyon virus (California serogroup) and the importance of maternal antibody in viral maintenance. J. Wildl. Dis. 23:12–22 [DOI] [PubMed] [Google Scholar]
- 20. Heath SE, Artsob H, Bell RJ, Harland RJ. 1989. Equine encephalitis caused by snowshoe hare (California serogroup) virus. Can. Vet. J. 30:669–671 [PMC free article] [PubMed] [Google Scholar]
- 21. Hustins S. 2006. Mosquito ecology in relation to land-use changes and potential West Nile virus in Newfoundland. M.S. thesis. Memorial University of Newfoundland, St. John's, Newfoundland and Labrador, Canada [Google Scholar]
- 22. Issel CJ. 1973. Isolation of Jamestown Canyon virus (a California group arbovirus) from a white-tailed deer. Am. J. Trop. Med. Hyg. 22:414–417 [DOI] [PubMed] [Google Scholar]
- 23. Langlois JP, Fahrig L, Merriam G, Artsob H. 2001. Landscape structure influences continental distribution of hantavirus in deer mice. Landsc. Ecol. 16:255–266 [Google Scholar]
- 24. Lynch JA, Binnington BD, Artsob H. 1985. California serogroup virus infection in a horse with encephalitis. J. Am. Vet. Med. Assoc. 186:389. [PubMed] [Google Scholar]
- 25. MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton University Press, Princeton, NJ [Google Scholar]
- 26. Makowski K, Dimitrova K, Andonova M, Drebot M. 2009. An overview of California serogroup virus diagnostics and surveillance in Canada in 2008. Int. J. Antimicrob. Agents 34(Suppl 2):S19 [Google Scholar]
- 27. McFarlane BL, et al. 1981. Antibodies to snowshoe hare virus of the California group in the snowshoe hare (Lepus americanus) and domestic animal populations of Prince Edward Island. Can. J. Microbiol. 27:1224–1227 [DOI] [PubMed] [Google Scholar]
- 28. McFarlane BL, Embree JE, Embil JA, Rosee KR, Artsob H. 1982. Antibodies to the California group of arboviruses in animal populations of New Brunswick. Can. J. Microbiol. 28(2):200–204 [DOI] [PubMed] [Google Scholar]
- 29. Mokry J, Artsob H, Butler R. 1984. Studies on California serogroup virus activity in Newfoundland, Canada. Mosquito News 44:310–314 [Google Scholar]
- 30. Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. 2008. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J. Med. Entomol. 45:1143–1151 [DOI] [PubMed] [Google Scholar]
- 31. Murdock CC, Olival KJ, Perkins SL. 2010. Molecular identification of host feeding patterns of snow-melt mosquitoes (Diptera: Culicidae): potential implications for the transmission ecology of Jamestown Canyon virus. J. Med. Entomol. 47:226–229 [DOI] [PubMed] [Google Scholar]
- 32. Nielson LT, Mokry JE. 1982. Mosquitoes of the island of Newfoundland—a report on new records and notes on the species. Mosquito Syst. 14:34–40 [Google Scholar]
- 33. Pickavance JR, Bennett GF, Phipps J. 1970. Some mosquitoes and blackflies from Newfoundland. Can. J. Zool. 48:621–624 [Google Scholar]
- 34. Rohwer F, Prangishvili D, Lindell D. 2009. Roles of viruses in the environment. Environ. Microbiol. 11:2271–2274 [DOI] [PubMed] [Google Scholar]
- 35. Sahu SP, et al. 2000. Isolation of Jamestown Canyon virus (California virus group) from vesicular lesions of a horse. J. Vet. Diagn. Invest. 12:80–83 [DOI] [PubMed] [Google Scholar]
- 36. Sax DF, Gaines SD, Brown JH. 2002. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and animals. Am. Nat. 160:766–783 [DOI] [PubMed] [Google Scholar]
- 37. Suttle CA. 2005. Viruses in the sea. Nature 437:356–361 [DOI] [PubMed] [Google Scholar]
- 38. Watts DM, LeDuc JW, Bailey CL, Dalrymple JM, Gargan TP. 1982. Serologic evidence of Jamestown Canyon and Keystone virus infection in vertebrates of the Delmarva peninsula. Am. J. Trop. Med. Hyg. 31:1245–1251 [DOI] [PubMed] [Google Scholar]
- 39. Wood DM, Dang PT, Ellis RA. 1979. The mosquitoes of Canada, Diptera: Culicidae. Agriculture Canada publication no. 1686. Agriculture Canada, Quebec, Canada [Google Scholar]
- 40. Woodrow EF, Heringa PK. 1987. Pedoclimatic zones of the island of Newfoundland. Report no. 32. Newfoundland Soil Survey, Agriculture Canada, Quebec, Canada [Google Scholar]
- 41. Zar JH. 1999. Biostatistical analyses. Prentice Hall, Upper Saddle River, NJ [Google Scholar]