Abstract
Objective
To investigate whether Fourier transform infrared imaging spectroscopy (FT-IRIS), a modality based on molecular vibrations, is a viable alternative to histology and immunohistochemistry for assessment of tissue quality and patient clinical outcome.
Methods
Osteochondral biopsies were obtained from patients (9–65 months post-surgery) who underwent an autologous chondrocyte implantation (ACI) procedure to repair a cartilage defect (N=14). The repair tissue was evaluated histologically by OsScore grading, for the presence of types I and II collagen by immunohistochemistry (IHC), and for proteoglycan distribution and collagen quality parameters by FT-IRIS. Patient clinical outcome was assessed by the Lysholm score.
Results
Improvement in Lysholm score occurred in 79% of patients. IHC staining showed the presence of types I and II collagen in all samples, with a greater amount of collagen type II in the deep zone. The amount and location of immunostaining for type II collagen correlated to the FT-IRIS-derived parameters of relative proteoglycan content and collagen helical integrity. In addition, the improvement in Lysholm score post-ACI correlated positively with the OsScore, type II collagen (IHC score) and FT-IRIS-determined parameters. Regression models for the relation between improvement in Lysholm score and either OsScore, IHC area score or the FT-IRIS parameters all reached significance (p < 0.01). However, the FT-IRIS model was not significantly improved with inclusion of the OsScore and IHC score parameters.
Conclusion
Demonstration of the correlation between FT-IRIS-derived molecular parameters of cartilage repair tissue and patient clinical outcome lays the groundwork for translation of this methodology to the clinical environment to aid in the management of cartilage disorders and their treatment.
Keywords: Cartilage Repair, Autologous Chondrocyte Implantation, Histology, Type II Collagen, Proteoglycan, FTIR Imaging Spectroscopy
Introduction
Damage or degeneration of cartilage is frequently associated with joint pain and with changes in the macromolecular structure and content of the primary cartilage components [1–5]. It is widely accepted that cartilage injuries do not heal spontaneously [1], which is related in part to the avascular nature of the tissue and low mitotic activity of chondrocytes [6]. A variety of approaches have been investigated to improve cartilage healing including microfracture [7], subchondral drilling [7], osteochondral grafting [8], bone-marrow stimulation and a one-step technique based on bone marrow-derived cell transplantation [9]. These techniques rely on the potential of non-differentiated cells located in the subchondral area to migrate into the defect region and to differentiate into active chondrocytes [10, 11]. More cell based methods for the repair of articular cartilage have also been developed on the basis of autologous cartilage–bone transplants or transplantation of cultured autologous chondrocytes [8, 12]. Autologous chondrocyte implantation (ACI) is being used increasingly to treat cartilage defects [13]. This procedure aims to stimulate autologous cells to synthesize the extracellular matrix components of articular cartilage and to generate a zonal structure similar to normal articular cartilage [14]. Brittberg et al [12] applied the ACI technique clinically for the first time with good to excellent results for healing. A number of studies followed with similar results, suggesting that ACI is an effective procedure for healing cartilage defects of the knee [1, 15–17].
Evaluation of the success of cartilage repair procedures can include assessment of tissue harvested from the repair site, and several scoring systems are in use for semi-quantitative histological tissue evaluations [18], including the ICRS II score [19], O’Driscoll score [20] and OsScore [21]. In general, these scoring systems consider tissue integrity, proteoglycan staining, chondrocyte clustering, presence of hyaline cartilage, and integration of repair with surrounding tissue. In addition, the presence of type II collagen in repair tissue is an important indicator of successful articular cartilage repair [22, 23], as mechanically inferior fibrocartilaginous tissue that contains type I collagen is often produced [24]. Therefore, immunohistochemical (IHC) assessment of types I and II collagen is often performed as well [14, 25]. The major drawback for both histological and IHC assessment is the requirement to harvest tissues for analysis, which limits these evaluations in a clinical scenario.
Fourier transform infrared imaging spectroscopy (FT-IRIS) has been used to characterize the structure, distribution and orientation of extracellular matrix molecules, including types I and II collagen and proteoglycan, in histological sections of connective tissues [26], and in particular, in diseased and regenerative or engineered cartilage [4, 27–34]. Further, the use of infrared spectroscopic techniques via fiber optic enables assessment of tissues in situ, without biopsy [35, 36]. Thus, the possibility of utilizing these methods to monitor cartilage repair tissue quality, and thereby assist in disease management, is attractive. In particular, if parameters derived from infrared spectral characterization of repair tissue correlate with traditional histology-derived scores or with patient clinical outcome, FT-IRIS would provide a means to non-destructively assess the tissue status and could perhaps predict success of the repair procedure.
To date, there has been limited success in correlating the clinical outcome of patients who have undergone cartilage repair procedures with the quality of the repair tissue formed [21, 23]. The aims of the current study were, therefore threefold, namely (I) to investigate the molecular properties of cartilage repair tissue post-ACI procedure using FT-IRIS, (II) to assess correlations between FT-IRIS-derived properties and the gold-standard techniques of histological grading and type II collagen IHC and (III) to investigate if FT-IRIS is a viable alternative to histological or immunochemical assessment by exploring the relationship between histological, IHC, or FT-IRIS assessment of tissue quality and clinical outcome.
Materials and Methods
Tissue
Patients, aged 28–53 years (N=14), with chondral or osteochondral defects on the femoral condyles or trochlea, were treated with ACI according to the technique of Brittberg et al [12]. Full-depth core biopsy samples (1.8 mm in diameter) were obtained from patients who had undergone ACI 9–65 months previously. In addition, tissue from a healthy cadaveric knee (40 year old) with macroscopically normal cartilage was obtained within 24 hours of death from the UK Human Tissue Bank. A biopsy was obtained from the medial femoral condyle, similar to the region most commonly treated with ACI. All tissues obtained were under approval of the local Research Ethical Committee. The tissues were immediately snap frozen and stored in liquid nitrogen until processed.
Clinical outcome (Lysholm score)
Patient clinical outcome was evaluated based on the Lysholm score [37, 38]. The Lysholm scale is a well-validated functional and pain score designed for knee injuries that has been utilized to assess the clinical outcome of ACI procedures [37, 39] and ranges from 0 (poor outcome) to 100 (best). The parameters that comprise the Lysholm score are summarized in Table 1 [40]. For each patient, the Lysholm score was assessed at the time of surgery (pre-operatively) and at the time of biopsy; the difference between these two values was reported as the actual improvement in Lysholm score, or percent Lysholm improvement.
Table 1.
Parameters evaluated in the Lysholm score (total 100 points) [40].
| Parameter | Point |
|---|---|
| Limp | 5 points |
| Support | 5 points |
| Stair climbing | 10 points |
| Squatting | 5 points |
| Instability | 25 points |
| Pain | 25 points |
| Swelling | 10 points |
| Locking | 15 points |
Histology and immunohistochemical (IHC) analysis
Seven micron thick cryosections of the harvested biopsies were stained with hematoxylin and eosin (H&E) for histologic evaluation. The semi-quantitative OsScore was utilized for assessment of tissue quality [21], and ranges from 0 (worst) to 10 (best), based on the following features of the repair tissue: cartilage type present (hyaline cartilage, fibrocartilage or fibrous tissue), integrity of the tissue surface, degree of metachromasia (toluidine blue or safranin O staining), formation of chondrocyte clusters, presence of blood vessels or mineralization, and integration with the calcified cartilage and underlying bone.
IHC staining was performed on cryosections using antibodies specific to types I or II collagen. Separate tissue sections were incubated for 1 h at room temperature with primary antibodies against type I collagen (monoclonal antihuman, clone no 1-8H5; ICN) and against type II collagen (CIICI; Developmental Studies Hybridoma Bank, Iowa, USA). The type II collagen stained IHC images were scored in two ways. A semi-quantitative assessment of the percentage of area of the section of cartilage tissue which was immunostained for type II collagen, termed ‘IHC Area score’, was obtained using image analysis software (NIS-Elements Basic Research, Nikon, Japan) [14]. A second more detailed assessment of the staining intensity in defined regions was also undertaken on a number of samples and used to determine the relationship of the IHC intensity (termed ‘IHC Intensity score’) with FT-IRIS derived parameters. For this, each tissue section was divided into 9 regions and each region graded from 0 to 3 based on staining intensity, where grade 0 represents the minimum amount of type II collagen staining (white) and grade 3 represents the maximum amount of type II collagen immunostaining (dark brown).
FT-IRIS data acquisition
FT-IRIS data were acquired in the mid-infrared region, 4000 – 800 cm−1, at 8 cm−1 spectral resolution and 25-μm spatial resolution, with 2 co-added scans per pixel, using a Spectrum Spotlight 400 FT-IR imaging spectrometer (Perkin Elmer, CT, USA). Polarized FT-IRIS data were collected with an infrared polarizer inserted in the beam path. Data collection time was approximately 30 minutes per sample.
FT-IRIS data processing
FT-IRIS images were created based on vibrational absorbances for the specific molecular components of interest in cartilage using ISys software v5.0 (Malvern Instrument, Columbia, MD) [28]. Several studies have utilized IR spectroscopy to assess the composition of cartilage, based on absorbances from different components. An earlier study from our lab where spectroscopic evaluation of mixtures of pure collagen and aggrecan (the major proteoglycan (PG) in cartilage) was performed demonstrated that the integrated area under the protein amide I band (1594–1718 cm−1) correlates with collagen content [26]. In the same study, it was shown that the integrated area of the infrared vibration from the sugar ring absorbance of PGs in the range of 985–1140 cm−1 correlates with PG content. Although more recent studies have shown some improvement in specificity of PG assessment using multivariate methodology [41], the PG parameters used in the current study have been validated by correlation to both biochemical and histology data [34, 42]. The ratio of the integrated area of the PG absorbance to the amide I collagen absorbance (PG/Am I) was evaluated to obtain the relative quantity and distribution of the PG component with respect to collagen. The area under the infrared absorbance centered at 1338 cm−1 (1326–1356 cm−1), a feature attributed to CH2 side-chain vibrations in collagen, has previously been shown to decrease in intensity as collagen denatures [35, 43], and thus is related to whether the collagen triple helix is intact. This absorbance was ratioed to the amide II band (1492–1594 cm−1) to evaluate the helical integrity of collagen, and defined as “collagen integrity” [43]. The area ratio of the amide I and amide II absorbances from collagen were used to create the images from the polarized data. It has previously been shown that this ratio can be used as an index of collagen fibril orientation [44]. The collagen fibril orientation was defined as: (i) an amide I/II polarized ratio ≥2.7 representing fibrils parallel to the articular surface, (ii) an amide I/II ratio ≤1.7 reflecting fibrils perpendicular to the articular surface, and (iii) amide I/II ratios between 2.7–1.7 indicative of random or mixed fibril orientation [44].
Statistical analysis
A Pearson correlation analysis was used to assess the relationship between the FT–IRIS data, Lysholm score improvement, histology OsScore and IHC data. Mean values, p-value and 95% confidence intervals (95% CI) were calculated for descriptive statistics. To assess whether FT-IRIS derived data have the potential to replace histological and immunohistochemical assessment, a multiple linear regression analysis was used to investigate the relationship between tissue quality as reflected in FT-IRIS-derived properties, OsScore, or IHC data, or a combination of these data and the clinical outcome (Lysholm score improvement). In this analysis, Lysholm score improvement was regarded as the dependent variable and all other variables as independent. The strength of the relationships was assessed based on R-squared (R2) values and root mean square errors (RMSEs) of the models from the regression analysis. An F-test was used to assess whether addition of extra variables improved the models significantly [45]. Significance was determined at p ≤ 0.05 for all analyses. All data were analyzed in Unscrambler X software (Camo, Norway) and Microsoft Excel 2010.
Results
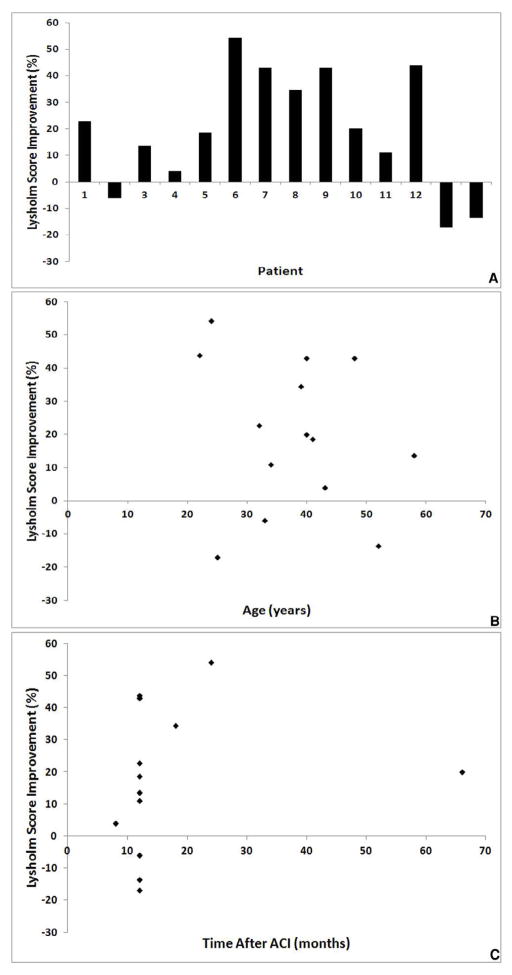
There was a wide range of change in Lysholm scores following treatment with ACI, with an improvement in Lysholm score occurring in 11 of the 14 patients (79%; Figure 1A). There was no significant correlation between the percentage improvement in Lysholm score and the age of the patient (Figure 1B, R = 0.1, 95% CI -0.43 to 0.58, p = 0.72), or time of biopsy (Figure 1C, R = 0.02, 95% CI -0.49 to 0.52, p = 0.94).
Fig. 1.
Percentage Lysholm score improvement 9–65 months post-ACI treatment A) For each individual patient, B) versus patient age, and C) versus time of biopsy.
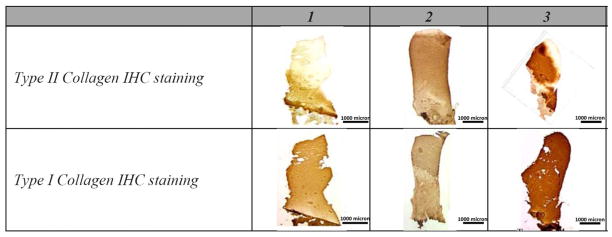
Immunohistochemical staining demonstrated the presence of types I and II collagen in the tissue samples (Figure 2). The type II collagen immunostaining typically occurred in the lower part of the biopsy core, close to the underlying bone, covering an average of 60% area of the cartilaginous section, while staining for type I collagen was present throughout the majority of the cores.
Fig. 2.
IHC staining for type I and II collagen antibodies from of a subset of ACI-treated biopsy samples.
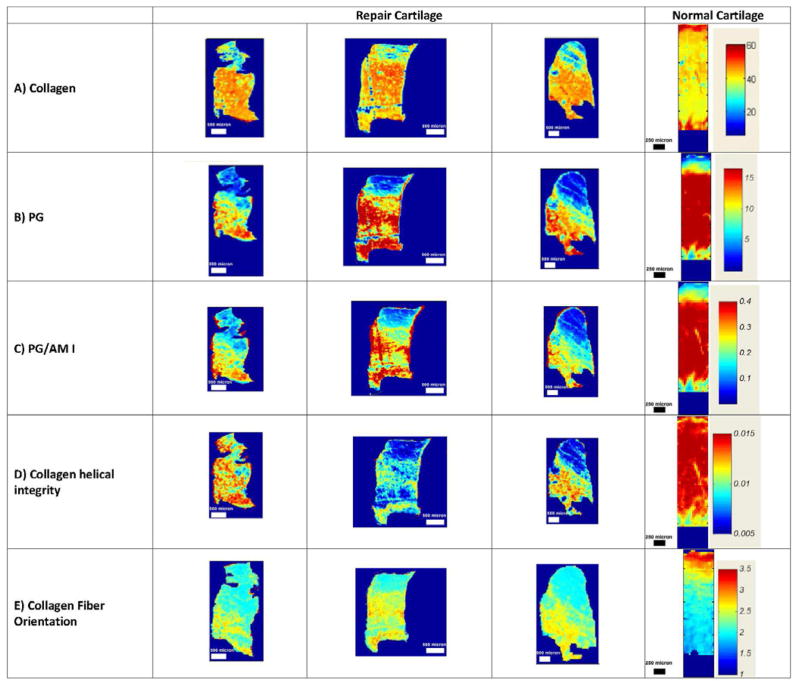
FT-IRIS analysis demonstrated that collagen (Figure 3A) and PG (Figure 3B, C) were also distributed zonally in the repair tissue, where more abundant collagen and PG were present in the middle and deep zones of the cartilage, respectively. Both PG and collagen content were higher in the normal cartilage sample compared to repair cartilage biopsies. Collagen helical integrity was also greater in the normal cartilage compared to repair tissue (Figure 3D). In contrast to normal cartilage, where the collagen fibrils were oriented parallel to the articular surface at the superficial zone, random in the middle, and perpendicular to the surface in the deep zone, there was no completely normal zonal distribution of collagen fibril orientation in the repair tissue samples (Figure 3E). Most repair biopsy samples showed the FT-IRIS parameter reflecting collagen fibers to be more randomly oriented through the full depth of tissue than in normal cartilage.
Fig. 3.
FT-IRIS images from a subset of ACI-treated biopsy samples and from normal cartilage. The red and dark blue in the color scales indicate the highest and lowest content, respectively, for A) collagen, B) PG, C) relative PG, D) collagen helical integrity, and E) collagen fibril orientation, where values less than 1.7 indicate fibers perpendicular to the surface, between 1.7 and 2.7 indicate randomly aligned fibers, and larger than 2.7 indicate collagen fibers with orientation parallel to the articular surface, respectively.
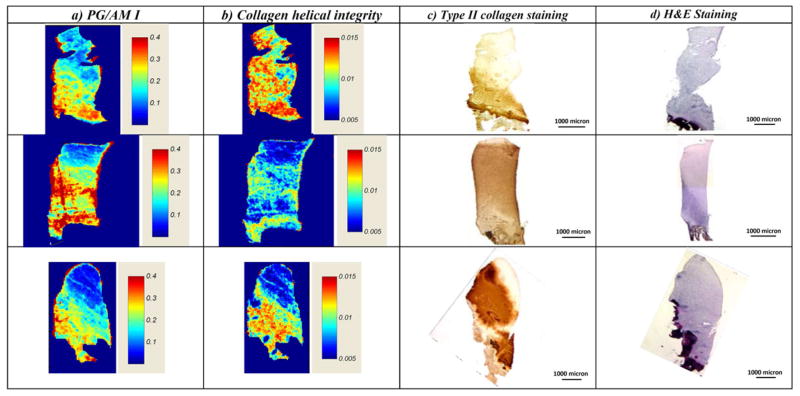
FT-IRIS derived PG/Am I integrated area ratio, indicative of the relative PG/collagen content of the repair tissue, was highest in the tissue adjacent to the bone for all samples (Figure 4A). Similarly, there were higher values for the collagen helical integrity in the areas near the bone than elsewhere in the repair tissue (Figure 4B). Qualitatively, the distribution of both these parameters was very similar to the distribution of type II collagen (Figure 4C).
Fig. 4.
FT-IRIS derived parameters from a subset of ACI-treated biopsy samples. A) PG/collagen content, and B) collagen helical integrity. Corresponding IHC image of C) collagen type II staining, and D) histology image of H & E staining.
Histology showed that generally, the surface zone was smooth and there was good basal integration between the repair tissue and the underlying bone (Figure 4D). OsScore values ranged from 2.5 to 9, with cartilage morphology being predominantly fibrocartilage in eight cores, hyaline cartilage in three cores, and a mixture of hyaline and fibrocartilage morphology in the remaining four cores.
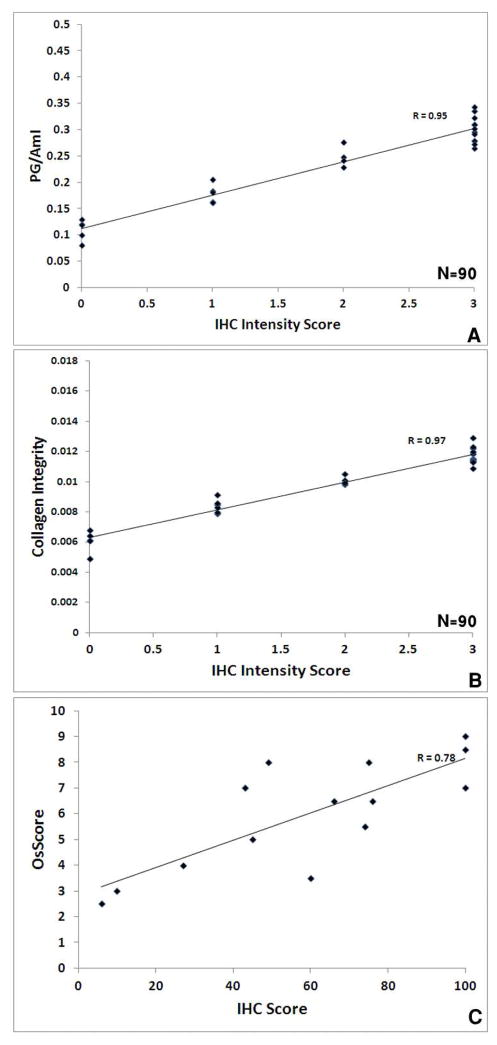
The IHC intensity score was significantly correlated with PG/Am I (R= 0.95, 95% CI 0.93 to 0.97, p<0.0001, Figure 5A), with collagen helical integrity (R= 0.97, 95% CI 0.96 to 0.98, p<0.0001, Figure 5B) and with the OsScore (R= 0.78, 95% CI 0.43 to 0.93, p=0.001, Figure 5C).
Fig. 5.
Correlation of IHC area and intensity scores for type II collagen with FT-IRIS-derived parameters and histology or immunohistochemistry scores; A) IHC intensity score vs. PG content, p < 0.0001 (95% CI: 0.93 to 0.97), B) IHC intensity score vs. collagen integrity, p < 0.0001 (95% CI: 0.96 to 0.98), C) IHC area score vs. OsScore, p = 0.001 (95% CI: 0.43 to 0.93).
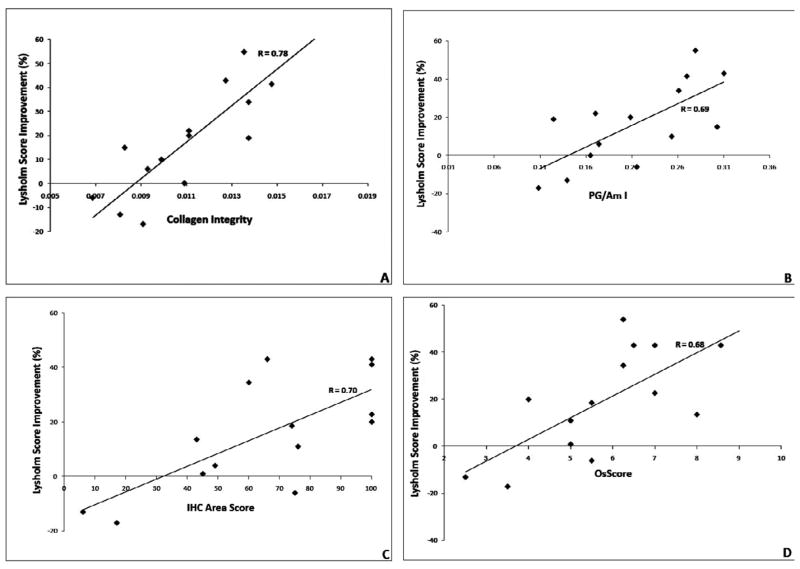
Percentage improvement in Lysholm score from pre to post-treatment (at the time of the biopsy) was significantly correlated with collagen helical integrity (R= 0.78, 95% CI 0.43 to 0.93, p=0.001, Figure 6A), PG/Am I (R= 0.69, 95% CI 0.25 to 0.89, p=0.006, Figure 6B), the IHC area score (R= 0.70, 95% CI 0.27 to 0.90, p=0.005, Figure 6C), and the OsScore (R= 0.68, 95% CI 0.25 to 0.88, p=0.007, Figure 6D).
Fig. 6.
Correlation of FT-IRIS derived parameters with percentage Lysholm score improvement and IHC area score values; A) Collagen integrity vs. percentage Lysholm score improvement, p = 0.001 (95% CI: 0.43 to 0.93), B) PG content vs. percentage Lysholm score improvement, p = 0.006 (95% CI: 0.25 to 0.89), C) IHC area score vs. percentage Lysholm score improvement, p = 0.004 (95% CI: 0.27 to 0.89), D) OsScore vs. percentage Lysholm score improvement, p = 0.006 (95% CI: 0.23 to 0.88).
In line with the correlation analysis, regression models for the relation between percentage Lysholm score improvement (LSI) as dependent and either OsScore, IHC area score or the FT-IRIS parameters as independent parameters all reached significance (Equation 1–3, Table 2). Once the information from the FT-IRIS parameters was taken into account, addition of the IHC area score, the OsScore or both, slightly increased the coefficient of determination (R2) of the relation between the independent parameters and the LSI (Equations 4 and 5, Table 2). However, this increase in R2 was not significant (p=0.07 or more; Table 2).
Table 2.
Regression analysis parameters for prediction of Lysholm score improvement with histology, IHC and FT-IRIS variables.
| Variables* | R | 95% CI of correlation | R2 | RMSE | p value (Regression) | p value (F-Test) |
|---|---|---|---|---|---|---|
| Histology | 0.68 | 0.23–0.88 | 0.48 | 6.60 | 0.006 | - |
| IHC | 0.70 | 0.27–0.89 | 0.5 | 7.07 | 0.004 | - |
| FT-IRIS | 0.81 | 0.49–0.93 | 0.66 | 4.52 | 0.002 | - |
| Histology + FT-IRIS | 0.82 | 0.51:0.94 | 0.67 | 4.88 | 0.003 | Compared to: Histology: 0.12 FT-IRIS: 0.11 |
| IHC+FT-IRIS | 0.84 | 0.55–0.94 | 0.71 | 4.72 | 0.004 | Compared to: IHC: 0.15 FT-IRIS: 0.11 |
| Histology + IHC+FT-IRIS | 0.85 | 0.58–0.95 | 0.72 | 3.63 | 0.013 | Compared to: Histology: 0.18 IHC: 0.094 FT-IRIS: 0.081 IHC+FT-IRIS: 0.07 |
Histology:
OsScore; IHC: Immunohistochemistry area score; FT-IRIS parameters: PG/Am I and collagen integrity; R = Correlation coefficient; R2= Coefficient of determination; RMSE = Root mean square error of prediction.
Equation 1: LSI = 9.22 ∗ OsScore − 33.89
Equation 2: LSI = 0.47 ∗ IHC area score - 15.17
Equation 3: LSI = 16.95 + 0.95∗ PG/Am I + 1.56∗ Collagen integrity
Equation 4: LSI = 12.72 + 0.51∗IHC area score + 0.325∗ PG/Am I + 1.57∗ Collagen integrity
Equation 5: LSI = 3.41 + 2.57∗OsScore + 0.4∗IHC area score + 0.325∗ PG/Am I + 0.968∗ Collagen integrity
Discussion
In the current study, we found that the ACI procedure improves clinical outcome as measured using the Lysholm score in the majority of patients. The repair tissue was generally composed of both type I and II collagen, with highest concentrations of type II collagen in the cartilage deep zone near to the calcified tissue. The type II collagen distribution was similar to the relative proteoglycan/collagen and helical integrity maps obtained from the FT-IRIS data. Parameters calculated from the FT-IRIS data correlated highly and significantly with the histological and immunohistochemical data as characterized by OsScore and IHC Area, respectively. All three also correlated highly with the clinical outcome characterized by increase in Lysholm score post-treatment. Multiple regression analysis showed that once the information from the FT-IRIS parameters was taken into account, information from neither the OsScore nor the IHC score could significantly improve the relation between FT-IRIS and clinical outcome.
Repair procedures for articular cartilage are particularly useful when the function of the joint can be significantly recovered and progression to joint degeneration can be inhibited or slowed [7]. It is important to monitor clinical progress, as well as tissue changes, post-treatment. In the current study, clinical improvement occurred in 79% of patients, similar to previous studies that reported a 65 to 90% success rate of ACI treatments [1, 15, 17]. However, the range of values for magnitude of improvement is large, underscoring both the subjective nature of assessment, and the heterogeneity of the population and procedure. Increased understanding of the molecular basis that underlies the clinical improvement could aid in the management of the repair process.
Immunostaining using antibodies that react with specific collagen types have been widely applied to assess collagen distribution in both normal and repair cartilage [46]. In the current study, immunopositivity for type I collagen occurred throughout the majority of the graft tissue, while type II collagen staining was primarily concentrated to the lower regions of the cartilage nearest the bone. Hence, a combination of type I and type II collagen was seen in all samples evaluated in this study. This reflects the presence of fibrocartilage, which, although not desirable, may go on to mature to hyaline cartilage in some of the repair tissue, a phenomenon that has been reported previously [14, 22, 25]. Since type II collagen is typically present in hyaline cartilage, it appears that the tissue remodeling may be originating from the cartilage deep zone, at the bone-cartilage interface. Nonetheless, a greater amount of collagen type II in this sample set was linked to a greater improvement in Lysholm score. Unfortunately, since histological analysis and immunostaining require actual tissue sections and corresponding removal of some repair tissue, it is unrealistic to expect these assays to be performed in a routine clinical procedure.
FT-IR imaging spectroscopy has been used in cartilage tissue assessment for over 15 years. The cartilage repair process has been evaluated by FT-IRIS in two pre-clinical studies in rabbit models by Kim et al [34], and by Terajima et al. [47]. In the Kim study, it was shown that FT-IRIS analysis combined with MRI T2 can be utilized to assess progression of the repair process at the molecular level as an important step towards improved monitoring of repair tissue maturation. In the Terajima study, FT-IRIS evaluation of repair tissue was compared to biochemical analysis of collagen cross-links and composition. Although the FT-IRIS parameters paralleled the biochemical collagen data, there was no correlation with a previously described FT-IRIS “crosslink” parameter validated for type I collagen [28] and biochemical crosslink data, likely due to the combination of types I and II collagen in the repair tissue. Clinically, cartilage repair tissue has not been evaluated by FT-IRIS extensively, although studies of degenerated human cartilage tissue have shown that FT-IR provides quantitative information on compositional changes of collagen and proteoglycan. In general, damaged tissue shows changes in collagen quantity and distribution, in collagen fiber orientation, and loss of PGs [4, 35, 48]. The similar correlation found between FT-IRIS-derived molecular parameters (of both relative proteoglycan/collagen content as well as the collagen helical integrity) with improvement in Lysholm score, compared to that seen with the IHC-determined type II collagen content or OsScore for histology evaluation, indicates the potential of FT-IRIS as a modality for assessing repair tissue. Moreover, our work suggests that immunohistochemistry and histology-derived scores provide very little additional information to that derived from the FT-IRIS parameters. It should be noted that recent studies have utilized other FT-IRIS determined parameters that can be more specific for assessment of PG content in cartilage under certain conditions [29, 41]. The data presented here reflect an assessment of PG relative to collagen, which is likely to be an important indicator of the quality of the repair tissue.
In addition to the data on the distribution of the macromolecular components of the repair tissue, FT-IRIS allows an assessment of orientation of collagen fibrils, which is an important indicator of tissue integrity and function [49]. In contrast to normal cartilage, the majority of collagen fibrils in the repair tissue showed a random alignment, with little zonal stratification. Further, collagen fibrils with an alignment parallel to the surface were found in the deeper regions, as opposed to on the surface, reflecting a suboptimal tissue organization, as assessed by FT-IRIS, regardless of collagen type. Collagen fiber orientation studies using polarized light microscope and magnetic resonance imaging have suggested that the collagen fibers in normal cartilage curve from being perpendicular to the surface in subchondral regions to being parallel to the surface at superficial regions [50–52], which is similar to the orientations found in the normal tissue analyzed in this study. Further, it has been shown that the orientation of collagen fibers is a key determinant of their functionality in cartilage [6]. Therefore, improving the zonal stratification in repair tissue would likely enhance the tissue quality. Interestingly, the collagen integrity parameter was greater in regions where type II collagen was more prevalent. Previous work on the origin of this molecular parameter found that a reduction in the ratio of the 1338 cm−1 absorbance to the amide II absorbance was related to type II collagen degradation [28]. However, assessment of the differences in absorbance in this spectral region between type I and type II collagen has not yet been investigated. Based on the current results, it is possible that changes in the spectral signature of the 1338 cm−1 peak from the collagen molecule can be used as an indicator of the presence of type II collagen in repair tissue.
Improvement in Lysholm score was significantly and positively correlated to the collagen helical integrity, to PG/collagen content, IHC area score and OsScore. Together, these data suggest that improvement in clinical outcome is related to the amount of hyaline cartilage in the repair tissue. Hyaline cartilage gives the desired biomechanical and viscoelastic properties to repair tissue which ultimately determines its quality, and the clinical outcome of cartilage repair process [39]. Henderson et al. have also found that improving the clinical outcome of ACI treatment is related to the amount of hyaline cartilage in repair tissue [53].
Correlations of FT-IRIS-determined PG composition to cartilage histology data based on Alcian blue and Safranin O staining have been demonstrated previously [4, 28, 41], but the current study is the first to show direct correlations between IHC-determined type II collagen and two different FT-IRIS parameters, relative PG/collagen content and collagen helical integrity. Thus, the present data demonstrate the feasibility of assessment of the quality of the repair tissue post-ACI using a spectroscopic method without the need for biochemical assays and histology or immunohistochemistry. Clearly, the ability of infrared spectroscopy to probe several macromolecules simultaneously makes this a very powerful tool for assessment of repair tissue.
In spite of the advantages of FT-IRIS in evaluating post-ACI repair tissue, there are also limitations to the use of this technique. Although we were able to assess tissue properties, there are some parameters that we cannot obtain information on, such as how well the repair tissue integrates laterally to surrounding normal tissue, and mechanical competence of the tissue. Here, with FTIR imaging spectroscopy, we were able to determine the tissue composition at relatively high pixel resolution (25 micron sampling). However, when transferring this methodology to an infrared fiber optic probe, the region sampled will be in the order of ~ 1 mm diameter [36], and thus only a few spectra per area of interest will be obtained. Further, the mid-infrared fiber optic probes that are commercially available acquire data in reflectance or attenuated total reflectance mode, which enables sampling of the tissue surface only. Nonetheless, infrared fiber optic probe spectra collected from the cartilage surface in degenerative cartilage have been shown to correlate to full-depth tissue composition, and as well as having the advantage of being non-destructive, also has the advantage of data acquisition in approximately one minute [36]. Using a near infrared probe, it would be possible to do full depth sampling, but data interpretation is not as well understood as that in mid infrared region [54]. Further development of the fiber optic technique for repair cartilage assessment would require additional validation, but such advances could result in an important prognostic tool to accurately predict the likelihood of treatment efficacy in a non-destructive manner.
Acknowledgments
Role of funding source
Financial support for this study was provided by National Institutes of Health and Arthritis Research UK. The study sponsors had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
This study was supported by NIH EB000744 and Arthritis Research UK (Grant numbers 18480 and 19429). We are grateful to Mrs. H Evans and Mrs. J. Menage for sample preparation and immunohistochemistry and Helen McCarthy for IHC area measurements.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Author contribution statement
All authors revised the manuscript critically for important intellectual content, and all authors approved the final version to be published. AH, SR, JBR and NP participated in the conception and design of the study. AH, SR, JHK, and NP participated in data analysis and interpretation. NP and SR supervised the study, and AH performed the experiments and acquired data. NP provided support and interpretation for FT-IRIS analyses. SR, JHK and JBR provided support and interpretation for ACI treatment clinical outcome, and SR and JBR provided support and interpretation for immunohistochemistry and histology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakamura N, Miyama T, Engebretsen L, Yoshikawa H, Shino K. Cell-Based Therapy in Articular Cartilage Lesions of the Knee. Arthroscopy. The Journal of Arthroscopic & Related Surgery. 2009;25:531–552. doi: 10.1016/j.arthro.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Hogue JH, Mersfelder TL. Pathophysiology and first-line treatment of osteoarthritis. Ann Pharmacother. 2002;36:679–686. doi: 10.1345/aph.1A132. [DOI] [PubMed] [Google Scholar]
- 3.Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000;43:2202–2210. doi: 10.1002/1529-0131(200010)43:10<2202::AID-ANR7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Bi XH, Yang X, Bostrom MPG, Camacho NP. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochimica Et Biophysica Acta-Biomembranes. 2006;1758:934–941. doi: 10.1016/j.bbamem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clinics in Sports Medicine. 2005;24:1. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 8.Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics. 1997;20:525–538. doi: 10.3928/0147-7447-19970601-08. [DOI] [PubMed] [Google Scholar]
- 9.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 10.Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. [The microfracture technic in the management of complete cartilage defects in the knee joint] Orthopade. 1999;28:26–32. doi: 10.1007/s001320050318. [DOI] [PubMed] [Google Scholar]
- 11.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170–176. [PubMed] [Google Scholar]
- 12.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 13.Batty L, Dance S, Bajaj S, Cole BJ. Autologous chondrocyte implantation: an overview of technique and outcomes. Anz Journal of Surgery. 2011;81:18–25. doi: 10.1111/j.1445-2197.2010.05495.x. [DOI] [PubMed] [Google Scholar]
- 14.Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. The Knee. 2009;16:398–404. doi: 10.1016/j.knee.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillogly SD, Voigbt M, Blackburn T. Treatment of Articular Cartilage Defects of the Knee With Autologous Chondrocyte Implantation. Journal of orthopaedic and sport physical therapy. 1998;28:241–251. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 16.Bhosale AM, Kuiper JH, Johnson WE, Harrison PE, Richardson JB. Midterm to long-term longitudinal outcome of autologous chondrocyte implantation in the knee joint: a multilevel analysis. Am J Sports Med. 2009;37(Suppl 1):131S–138S. doi: 10.1177/0363546509350555. [DOI] [PubMed] [Google Scholar]
- 17.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Rutgers M, van Pelt MJP, Dhert WJA, Creemers LB, Saris DBF. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis and Cartilage. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A New Histology Scoring System for the Assessment of the Quality of Human Cartilage Repair: ICRS II. American Journal of Sports Medicine. 2010;38:880–890. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 20.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017–1035. [PubMed] [Google Scholar]
- 21.Roberts S, McCall IW, Darby AJ, Menage J, Evans H, Harrison PE, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60–73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12:1787–1798. doi: 10.1089/ten.2006.12.1787. [DOI] [PubMed] [Google Scholar]
- 23.Saris DBF, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. American Journal of Sports Medicine. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 24.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clinical Orthopaedics and Related Research. 1999;365:149–162. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Roberts S, Hollander AP, Caterson B, Menage J, Richardson JB. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis and Rheumatism. 2001;44:2586–2598. doi: 10.1002/1529-0131(200111)44:11<2586::aid-art439>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Camacho NP, West P, Torzilli PA, Mendelsohn R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62:1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 27.Baykal D, Irrechukwu O, Lin PC, Fritton K, Spencer RG, Pleshko N. Nondestructive assessment of engineered cartilage constructs using near-infrared spectroscopy. Appl Spectrosc. 2011;64:1160–1166. doi: 10.1366/000370210792973604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boskey A, Camacho NP. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28:2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang EY, Rieppo J. Enhancing FTIR imaging capabilities with two-dimensional correlation spectroscopy (2DCOS): A study of concentration gradients of collagen and proteoglycans in human patellar cartilage. Journal of Molecular Structure. 2006;799:196–203. [Google Scholar]
- 30.Xia Y, Ramakrishnan N, Bidthanapally A. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI) Osteoarthritis and Cartilage. 2007;15:780–788. doi: 10.1016/j.joca.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M, Kraft JJ, Volk AC, Pugarelli J, Pleshko N, Dodge GR. Characterization of a cartilage-like engineered biomass using a self-aggregating suspension culture model: Molecular composition using FT-IRIS. J Orthop Res. 2011:1–7. doi: 10.1002/jor.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irrechukwu ON, Lin PC, Fritton K, Doty S, Pleshko N, Spencer RG. Magnetic resonance studies of macromolecular content in engineered cartilage treated with pulsed low-intensity ultrasound. Tissue Eng Part A. 2011;17:407–415. doi: 10.1089/ten.tea.2010.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Bi X, Horton WE, Spencer RG, Camacho NP. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: histologic and biochemical correlations. J Biomed Opt. 2005;10:031105. doi: 10.1117/1.1922329. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Foo LF, Uggen C, Lyman S, Ryaby JT, Moynihan DP, et al. Evaluation of early osteochondral defect repair in a rabbit model utilizing fourier transform-infrared imaging spectroscopy, magnetic resonance imaging, and quantitative T2 mapping. Tissue Eng Part C Methods. 2010;16:355–364. doi: 10.1089/ten.tec.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West PA, Bostrom MP, Torzilli PA, Camacho NP. Fourier transform infrared spectral analysis of degenerative cartilage: an infrared fiber optic probe and imaging study. Appl Spectrosc. 2004;58:376–381. doi: 10.1366/000370204773580194. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Thomson M, Dicarlo E, Yang X, Nestor B, Bostrom MP, et al. A chemometric analysis for evaluation of early-stage cartilage degradation by infrared fiber-optic probe spectroscopy. Appl Spectrosc. 2005;59:1527–1533. doi: 10.1366/000370205775142593. [DOI] [PubMed] [Google Scholar]
- 37.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 38.Smith HJ, Richardson JB, Tennant A. Modification and validation of the Lysholm Knee Scale to assess articular cartilage damage. Osteoarthritis and Cartilage. 2009;17:53–58. doi: 10.1016/j.joca.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 40.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985:43–49. [PubMed] [Google Scholar]
- 41.Rieppo L, Saarakkala S, Narhi T, Holopainen J, Lammi M, Helminen HJ, et al. Quantitative Analysis of Spatial Proteoglycan Content in Articular Cartilage With Fourier Transform Infrared Imaging Spectroscopy: Critical Evaluation of Analysis Methods and Specificity of the Parameters. Microscopy Research and Technique. 2010;73:503–512. doi: 10.1002/jemt.20789. [DOI] [PubMed] [Google Scholar]
- 42.Kim M, Bi XH, Horton WE, Spencer RG, Camacho NP. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: histologic and biochemical correlations. Journal of Biomedical Optics. 2005;10:1–6. doi: 10.1117/1.1922329. [DOI] [PubMed] [Google Scholar]
- 43.West PA, Torzilli PA, Chen C, Lin P, Camacho NP. Fourier transform infrared imaging spectroscopy analysis of collagenase-induced cartilage degradation. J Biomed Opt. 2005;10:14015. doi: 10.1117/1.1854131. [DOI] [PubMed] [Google Scholar]
- 44.Bi X, Li G, Doty SB, Camacho NP. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT-IRIS) Osteoarthritis and Cartilage. 2005;13:1050–1058. doi: 10.1016/j.joca.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Jamshidian M, Jennrich RI, Liu W. A study of partial F tests for multiple linear regression models. Computational Statistics & Data Analysis. 2007;51:6269–6284. [Google Scholar]
- 46.Jimenez SA, AlaKokko L, Prockop DJ, Merryman CF, Shepard N, Dodge GR. Characterization of human type II procollagen and collagen-specific antibodies and their application to the study of human type II collagen processing and ultrastructure. Matrix Biology. 1997;16:29–39. doi: 10.1016/s0945-053x(97)90114-1. [DOI] [PubMed] [Google Scholar]
- 47.Terajima M, Damle S, Penmatsa M, West P, Yang X, Bostrom M, et al. Temporal changes in collagen cross-links in spontaneous articular cartilage repair. Cartilage. doi: 10.1177/1947603512437736. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David-Vaudey E, Burghardt A, Keshari K, Brouchet A, Ries M, Majumdar S. Fourier Transform Infrared Imaging of focal lesions in human osteoarthritic cartilage. Eur Cell Mater. 2005;10:51–60. doi: 10.22203/ecm.v010a06. discussion 60. [DOI] [PubMed] [Google Scholar]
- 49.Korhonen RK, Julkunen P, Wilson W, Herzog W. Importance of collagen orientation and depthdependent fixed charge densities of cartilage on mechanical behavior of chondrocytes. J Biomech Eng. 2008;130:021003. doi: 10.1115/1.2898725. [DOI] [PubMed] [Google Scholar]
- 50.de Visser SK, Crawford RW, Pope JM. Structural adaptations in compressed articular cartilage measured by diffusion tensor imaging. Osteoarthritis Cartilage. 2008;16:83–89. doi: 10.1016/j.joca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 52.Changoor A, Tran-Khanh N, Methot S, Garon M, Hurtig MB, Shive MS, et al. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthritis Cartilage. 2011;19:126–135. doi: 10.1016/j.joca.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253–261. doi: 10.1097/01.blo.0000238829.42563.56. [DOI] [PubMed] [Google Scholar]
- 54.Spahn G, Plettenberg H, Kahl E, Klinger HM, Muckley T, Hofmann GO. Near-infrared (NIR) spectroscopy. A new method for arthroscopic evaluation of low grade degenerated cartilage lesions. Results of a pilot study. BMC Musculoskelet Disord. 2007;8:47. doi: 10.1186/1471-2474-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]