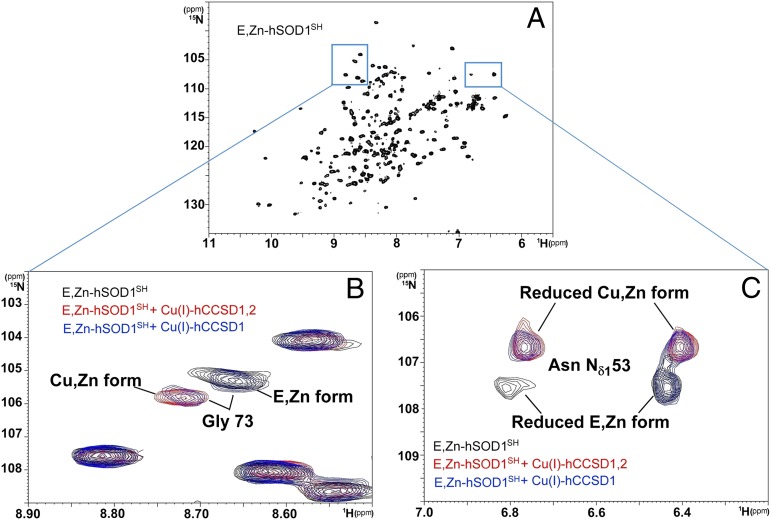
Fig. 2.
Transfer of Cu(I) to the hSOD1 is complete in the presence of Cu(I)-hCCSD1,2 and incomplete in the presence of Cu(I)-hCCSD1, with both proteins unable to promote the formation of the disulfide bond of hSOD1. (A) 1H-15N heteronuclear single quantum correlation spectroscopy (HSQC) spectrum of E,Zn-hSOD1SH. (B and C) Overlay of selected regions of the 1H-15N HSQC spectra of E,Zn-hSOD1SH (black), E,Zn-hSOD1SH in the presence of Cu(I)-hCCSD1,2 (red), and E,Zn-hSOD1SH in the presence of Cu(I)-hCCSD1 (blue). The chemical shift assignments of E,Zn-hSOD1SH and Cu,Zn-hSOD1S-S are available (42, 49). In B, the amide signal of residue Gly-73 is demonstrative of copper binding because it is located in proximity to the catalytic metal-binding site, whereas in C, the chemical shifts of side chain signals of Asn-53 are affected by both copper binding and oxidation. Asn-53 is located in loop 4 of hSOD1 and faces the C57-C146 disulfide on its formation.