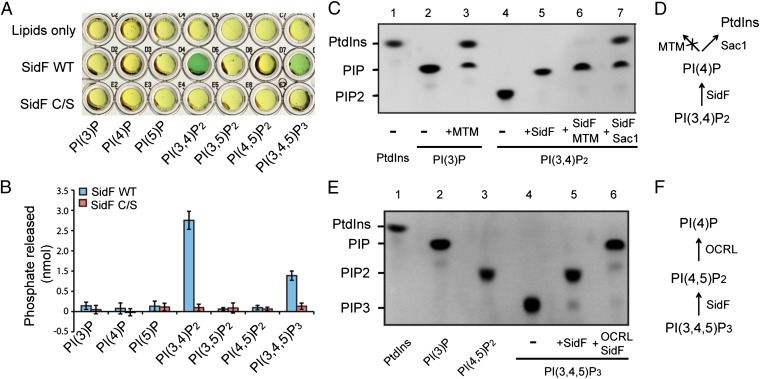
Fig. 1.
Legionella effector SidF is a phosphoinositide phosphatase. (A) Phosphoinositide substrate specificity of purified wild-type and C645S mutant SidF as determined by the malachite green assay (green color indicates the release of free phosphate). PI(3,4)P2 and PI(3,4,5)P3 are the preferred substrates. (B) Quantification of the amount of released phosphates. Data are from three replicate experiments (mean ± SEM). (C) Determination of SidF substrate specificity by fluorescent lipids. Phosphatase reactions were carried out with di-C8- Bodipy-FL-PI(3,4)P2 and PI phosphatases as labeled. In lane 6 and 7, the reactions were first carried out with SidF, and the products were further hydrolyzed by the addition of a specific 3-phosphatase MTM (lane 6) or Sac1 (lane 7), that hydrolyzes both PI(3)P and PI(4)P. (D) Schematic diagram to illustrate the enzymatic reactions shown in C. (E) TLC results of the hydrolysis of PI(3,4,5)P3 by SidF. In lane 6, the reaction was first carried out with SidF, and the products were further hydrolyzed by the addition of OCRL, a 5-phosphatase that hydrolyzes PI(4,5)P2. (F) Schematic illustration of the reactions in E.